Machine learning-based detection of label-free cancer stem-like cell fate
- PMID: 36352045
- PMCID: PMC9646748
- DOI: 10.1038/s41598-022-21822-z
Machine learning-based detection of label-free cancer stem-like cell fate
Abstract
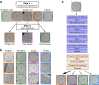
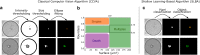
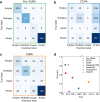
The detection of cancer stem-like cells (CSCs) is mainly based on molecular markers or functional tests giving a posteriori results. Therefore label-free and real-time detection of single CSCs remains a difficult challenge. The recent development of microfluidics has made it possible to perform high-throughput single cell imaging under controlled conditions and geometries. Such a throughput requires adapted image analysis pipelines while providing the necessary amount of data for the development of machine-learning algorithms. In this paper, we provide a data-driven study to assess the complexity of brightfield time-lapses to monitor the fate of isolated cancer stem-like cells in non-adherent conditions. We combined for the first time individual cell fate and cell state temporality analysis in a unique algorithm. We show that with our experimental system and on two different primary cell lines our optimized deep learning based algorithm outperforms classical computer vision and shallow learning-based algorithms in terms of accuracy while being faster than cutting-edge convolutional neural network (CNNs). With this study, we show that tailoring our deep learning-based algorithm to the image analysis problem yields better results than pre-trained models. As a result, such a rapid and accurate CNN is compatible with the rise of high-throughput data generation and opens the door to on-the-fly CSC fate analysis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Icha, J., Weber, M., Waters, J. C. & Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. Bioessays 39, 10.1002/bies.201700003 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

