Severity of neonatal influenza infection is driven by type I interferon and oxidative stress
- PMID: 36352099
- PMCID: PMC9724789
- DOI: 10.1038/s41385-022-00576-x
Severity of neonatal influenza infection is driven by type I interferon and oxidative stress
Abstract
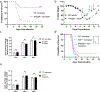
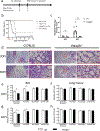
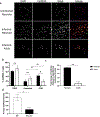
Neonates exhibit increased susceptibility to respiratory viral infections, attributed to inflammation at the developing pulmonary air-blood interface. IFN I are antiviral cytokines critical to control viral replication, but also promote inflammation. Previously, we established a neonatal murine influenza virus (IV) model, which demonstrates increased mortality. Here, we sought to determine the role of IFN I in this increased mortality. We found that three-day-old IFNAR-deficient mice are highly protected from IV-induced mortality. In addition, exposure to IFNβ 24 h post IV infection accelerated death in WT neonatal animals but did not impact adult mortality. In contrast, IFN IIIs are protective to neonatal mice. IFNβ induced an oxidative stress imbalance specifically in primary neonatal IV-infected pulmonary type II epithelial cells (TIIEC), not in adult TIIECs. Moreover, neonates did not have an infection-induced increase in antioxidants, including a key antioxidant, superoxide dismutase 3, as compared to adults. Importantly, antioxidant treatment rescued IV-infected neonatal mice, but had no impact on adult morbidity. We propose that IFN I exacerbate an oxidative stress imbalance in the neonate because of IFN I-induced pulmonary TIIEC ROS production coupled with developmentally regulated, defective antioxidant production in response to IV infection. This age-specific imbalance contributes to mortality after respiratory infections in this vulnerable population.
© 2022. The Author(s), under exclusive licence to Society for Mucosal Immunology.
Conflict of interest statement
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
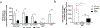

References
-
- Resch B, Kurath-Koller S, Eibisberger M, Zenz W, Prematurity and the burden of influenza and respiratory syncytial virus disease. World J Pediatr 12, 8–18 (2016). - PubMed
-
- Dawood FS et al. , Burden of seasonal influenza hospitalization in children, United States, 2003 to 2008. J Pediatr 157, 808–814 (2010). - PubMed
-
- Siegrist CA, Neonatal and early life vaccinology. Vaccine 19, 3331–3346 (2001). - PubMed
-
- Mohr E, Siegrist CA, Vaccination in early life: standing up to the challenges. Curr Opin Immunol 41, 1–8 (2016). - PubMed
-
- Adkins B, Leclerc C, Marshall-Clarke S, Neonatal adaptive immunity comes of age. Nat Rev Immunol 4, 553–564 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

