Protocol for the ORION trial (RadiO fRequency ablatION for haemorrhoids): a randomised controlled trial
- PMID: 36352146
- PMCID: PMC9839800
- DOI: 10.1007/s10151-022-02724-8
Protocol for the ORION trial (RadiO fRequency ablatION for haemorrhoids): a randomised controlled trial
Abstract
Background: Haemorrhoids are common and can significantly impact the personal and working lives of individuals. Those with more severe symptoms and those not responding to conservative management may require surgery. Current surgical techniques are associated with a degree of postoperative discomfort which may delay return to normal activity. Recurrence is lower in more radical procedures but resulting pain is higher. Radiofrequency ablation (RFA) is a new technique that is gaining popularity and has several hypothesised benefits, including reduced pain and recurrence. However, available evidence is limited. A recent overview from the National Institute for Health and Clinical Excellence recommended more research, in the form of randomised controlled trials, be carried out before further investment is made by national health services. Our aim is to assess whether RFA is at least as good in terms of recurrence as existing surgical interventions, but superior in terms of pain, for patients with symptomatic grade II and III haemorrhoids.
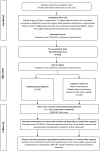
Methods: The RadiO fRequency ablatION for haemorrhoids (ORION) trial will be a pragmatic multicentre patient/assessor-blind parallel group-controlled trial with economic evaluation. The target sample size is 376 participants (188 per arm) and is based on two co-primary endpoints: (i) a non-inferiority design for recurrence and (ii) superiority design for pain at seven days. Participants with grade II or III haemorrhoids will be recruited in 16 National Health Service hospitals and randomised (1:1) to either RFA or surgeon's choice of surgery.
Conclusions: Results will inform future practice for the treatment of grade II-III haemorrhoids and provide evidence for national health services on future investments in RFA.
Trial registration: ISRCTN14474552.
Keywords: Haemorrhoids; Radiofrequency ablation; Randomised controlled trial; Surgical procedure.
© 2022. The Author(s).
Conflict of interest statement
No conflicts of interest to declare.
Figures
References
-
- Venara A, Podevin J, Godeberge P, et al. A comparison of surgical devices for grade II and III hemorrhoidal disease. Results from the LigaLongo Trial comparing transanal Doppler-guided hemorrhoidal artery ligation with mucopexy and circular stapled hemorrhoidopexy. Int J Colorectal Dis. 2018;33:1479–1483. doi: 10.1007/S00384-018-3093-8. - DOI - PubMed
-
- Watson AJM, Hudson J, Wood J, et al. Comparison of stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease (eTHoS): a pragmatic, multicentre, randomised controlled trial. Lancet. 2016;388:2375–2385. doi: 10.1016/S0140-6736(16)31803-7/ATTACHMENT/82EEAE74-5E13-42C3-8974-70FADFD73DC7/MMC1.PDF. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical