Gpr83 Tunes Nociceptor Function, Controlling Pain
- PMID: 36352334
- PMCID: PMC10119354
- DOI: 10.1007/s13311-022-01327-3
Gpr83 Tunes Nociceptor Function, Controlling Pain
Abstract
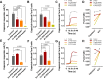
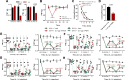
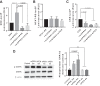
The function of peripheral nociceptors is frequently tuned by the action of G protein-coupled receptors (GPRs) that are expressed in them, which contribute to pain alteration. Expanding new information on such GPRs and predicting their potential outcomes can help to construct new analgesic strategies based on their modulations. In this context, we attempted to present a new GPR not yet acknowledged for its pain association. Gpr83 exhibits relatively high expressions in the peripheral nervous system compared to other tissues when we mined and reconstructed Gene Expression Omnibus (GEO) metadata, which we confirmed using immunohistochemistry on murine dorsal root ganglia (DRG). When Gpr83 expression was silenced in DRG, neuronal and behavioral nociception were all downregulated. Pathologic pain in hind paw inflammation and chemotherapy-induced peripheral neuropathy were also alleviated by this Gpr83 knockdown. Dependent on exposure time, the application of a known endogenous Gpr83 ligand PEN showed differential effects on nociceptor responses in vitro. Localized PEN administration mitigated pain in vivo, probably following Gq/11-involved GPR downregulation caused by the relatively constant exposure. Collectively, this study suggests that Gpr83 action contributes to the tuning of peripheral pain sensitivity and thus indicates that Gpr83 can be among the potential GPR targets for pain modulation.
Keywords: Analgesia; Gpr83; Nociceptor; PEN; Pain.
© 2022. The American Society for Experimental Neurotherapeutics, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

