Prevalence of hepatotoxicity among HIV-infected patients in Ethiopia: a systematic review and meta-analysis
- PMID: 36352398
- PMCID: PMC9647905
- DOI: 10.1186/s12879-022-07838-w
Prevalence of hepatotoxicity among HIV-infected patients in Ethiopia: a systematic review and meta-analysis
Abstract
Background: Globally, the human immunodeficiency virus has been recognized as a major public health concern. The direct toxicity of antiretroviral medicines or their active metabolites causes liver cell destruction by different mechanisms, inducing immune-mediated inflammation, oxidative stress, and other mechanisms. On the other hand, the virus itself also produces hepatotoxicity. Therefore, this systematic review and meta-analysis aimed to assess the pooled prevalence of hepatotoxicity among HIV-infected patients in Ethiopia.
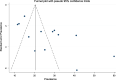
Methods: PubMed, Science Direct, Cochrane Library, Web of Science, and ResearchGate databases were used to find relevant articles. As well, various professional associations were searched to retrieve grey literature. The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of recruited studies. The data were extracted using Microsoft Excel, and the meta-analysis was carried out using STATA 14 software. I2 and Cochran's Q test were employed to assess the presence of heterogeneity between studies. A random effect model was used. The funnel plot and Egger's statistics were used to assess publication bias. Moreover, subgroup analysis and sensitivity analysis were also done.
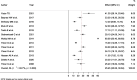
Results: The pooled prevalence of hepatotoxicity among HIV patients in Ethiopia was 25.45% (95% CI = 20.06-30.84%). There was high heterogeneity, with an I2 value of 93.7%. Subgroup analysis by HAART status showed a higher pooled prevalence of hepatotoxicity among HIV patients taking HAART (23.63%) than among HAART naive patients (7.29%). In subgroup analysis, the pooled prevalence of hepatotoxicity among HIV/Tb co-infected and HIV mono-infected patients was 26.3% and 17.94%, respectively.
Conclusion: The current systematic review and meta-analysis showed a high prevalence of hepatotoxicity among HIV-infected patients. Therefore, regular monitoring of hepatotoxicity among HIV-infected patients is required in order to avoid liver damage and other complications. Systematic review registration PROSPERO (2022:CRD42022334704).
Keywords: Antiretroviral therapy; Ethiopia; HIV/AIDS; Hepatotoxicity; Liver enzyme elevation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no potential competing interests.
Figures





References
-
- Okolie MN, Eghafona NO, Omoregie R. Anti-human immunodeficiency virus agents. J Med Lab Sci. 2003;12(1):1–14.
-
- De Cock KM, Mbori-Ngacha D, Marum E. Shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. The Lancet. 2002;360(9326):67–72. - PubMed
-
- Adebisi YA, Rabe A, Ekpenyong A, Okereke M, Sarah OA. Towards 90–90-90 target: COVID-19 and HIV response in Africa. J Infect Dis Epidemiol. 2021;7:191.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

