The transfer of maternal antibodies and dynamics of maternal and natural infection-induced antibodies against coxsackievirus A16 in Chinese children 0-13 years of age: a longitudinal cohort study
- PMID: 36352415
- PMCID: PMC9645321
- DOI: 10.1186/s12916-022-02604-w
The transfer of maternal antibodies and dynamics of maternal and natural infection-induced antibodies against coxsackievirus A16 in Chinese children 0-13 years of age: a longitudinal cohort study
Abstract
Background: A major hand-foot-and-mouth disease (HFMD) pathogen, coxsackievirus A16 (CVA16), has predominated in several of the last 10 years and caused the largest number of HFMD outbreaks between 2011 and 2018 in China. We evaluated the efficacy of maternal anti-CVA16 antibody transfer via the placenta and explored the dynamics of maternal and natural infection-induced neutralizing antibodies in children.
Methods: Two population-based longitudinal cohorts in southern China were studied during 2013-2018. Participants were enrolled in autumn 2013, including 2475 children aged 1-9 years old and 1066 mother-neonate pairs, and followed for 3 years. Blood/cord samples were collected for CVA16-neutralizing antibody detection. The maternal antibody transfer efficacy, age-specific seroprevalence, geometric mean titre (GMT) and immune response kinetics were estimated.
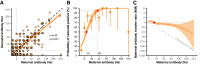
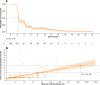
Results: The average maternal antibody transfer ratio was 0.88 (95% CI 0.80-0.96). Transferred maternal antibody levels declined rapidly (half-life: 2.0 months, 95% CI 1.9-2.2 months). The GMT decayed below the positive threshold (8) by 1.5 months of age. Due to natural infections, it increased above 8 after 1.4 years and reached 32 by 5 years of age, thereafter dropping slightly. Although the average duration of maternal antibody-mediated protection was < 3 months, the duration extended to 6 months on average for mothers with titres ≥ 64.
Conclusions: Anti-CVA16 maternal antibodies are efficiently transferred to neonates, but their levels decline quickly. Children aged 0-5 years are the main susceptible population and should be protected by CVA16 vaccination, with the optimal vaccination time between 1.5 months and 1 year of age.
Keywords: Antibody kinetics; Coxsackievirus A16 (CVA16); Maternal antibody; Natural infection; Transplacental transfer.
© 2022. The Author(s).
Conflict of interest statement
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang and Shanghai Roche Pharmaceutical Company. None of the research funding is related to this work. All other authors report no competing interests.
Figures


Similar articles
-
The transfer and decay of maternal antibodies against enterovirus A71, and dynamics of antibodies due to later natural infections in Chinese infants: a longitudinal, paired mother-neonate cohort study.Lancet Infect Dis. 2021 Mar;21(3):418-426. doi: 10.1016/S1473-3099(20)30480-1. Epub 2020 Oct 5. Lancet Infect Dis. 2021. PMID: 33031750
-
Comparison of Neutralizing Antibody Response Kinetics in Patients with Hand, Foot, and Mouth Disease Caused by Coxsackievirus A16 or Enterovirus A71: A Longitudinal Cohort Study of Chinese Children, 2017-2019.J Immunol. 2022 Jul 15;209(2):280-287. doi: 10.4049/jimmunol.2200143. Epub 2022 Jul 1. J Immunol. 2022. PMID: 35777850
-
Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008.Virol J. 2010 Nov 4;7:300. doi: 10.1186/1743-422X-7-300. Virol J. 2010. PMID: 21050463 Free PMC article.
-
Development and evaluation of an inactivated coxsackievirus A16 vaccine in gerbils.Emerg Microbes Infect. 2022 Dec;11(1):1994-2006. doi: 10.1080/22221751.2022.2093132. Emerg Microbes Infect. 2022. PMID: 35787233 Free PMC article.
-
Seroprevalence and Virologic Surveillance of Enterovirus 71 and Coxsackievirus A6, United Kingdom, 2006-2017.Emerg Infect Dis. 2021 Sep;27(9):2261-2268. doi: 10.3201/eid2709.204915. Emerg Infect Dis. 2021. PMID: 34423767 Free PMC article. Review.
Cited by
-
Epidemiological trends of hand, foot, and mouth disease in children under age 10, Jiangning District, Jiangsu, China (2009-2023).BMC Infect Dis. 2025 Jul 2;25(1):886. doi: 10.1186/s12879-025-11281-y. BMC Infect Dis. 2025. PMID: 40604541 Free PMC article.
-
Age-time-specific transmission of hand-foot-and-mouth disease enterovirus serotypes in Vietnam: A catalytic model with maternal immunity.Epidemics. 2024 Mar;46:100754. doi: 10.1016/j.epidem.2024.100754. Epub 2024 Feb 27. Epidemics. 2024. PMID: 38428358 Free PMC article.
-
Emergence and circulation of enterovirus B species in infants in southern China: A multicenter retrospective analysis.Virulence. 2024 Dec;15(1):2329569. doi: 10.1080/21505594.2024.2329569. Epub 2024 Mar 31. Virulence. 2024. PMID: 38555521 Free PMC article.
-
Reduced anti-viral IgG repertoire in HIV-exposed but uninfected infants compared to HIV-unexposed infants.iScience. 2024 Jun 15;27(7):110282. doi: 10.1016/j.isci.2024.110282. eCollection 2024 Jul 19. iScience. 2024. PMID: 39040054 Free PMC article.
-
Exploring the Birthday Week Effect on Hand, Foot, and Mouth Disease in Yunnan Province, China, From 2008 to 2022: Surveillance Data Analysis.JMIR Public Health Surveill. 2024 Sep 9;10:e59237. doi: 10.2196/59237. JMIR Public Health Surveill. 2024. PMID: 39250185 Free PMC article.
References
-
- Zhang Z, Liu Y, Liu F, Ren M, Nie T, Cui J, Chang Z, Li Z. Basic Reproduction Number of Enterovirus 71 and Coxsackievirus A16 and A6: Evidence From Outbreaks of Hand, Foot, and Mouth Disease in China Between 2011 and 2018. Clin Infect Dis. 2021;73(9):e2552–e2559. doi: 10.1093/cid/ciaa1853. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

