Cross-generational bacterial strain transfer to an infant after fecal microbiota transplantation to a pregnant patient: a case report
- PMID: 36352460
- PMCID: PMC9647999
- DOI: 10.1186/s40168-022-01394-w
Cross-generational bacterial strain transfer to an infant after fecal microbiota transplantation to a pregnant patient: a case report
Abstract
Background: Fecal microbiota transplantation (FMT) effectively prevents the recurrence of Clostridioides difficile infection (CDI). Long-term engraftment of donor-specific microbial consortia may occur in the recipient, but potential further transfer to other sites, including the vertical transmission of donor-specific strains to future generations, has not been investigated. Here, we report, for the first time, the cross-generational transmission of specific bacterial strains from an FMT donor to a pregnant patient with CDI and further to her child, born at term, 26 weeks after the FMT treatment.
Methods: A pregnant woman (gestation week 12 + 5) with CDI was treated with FMT via colonoscopy. She gave vaginal birth at term to a healthy baby. Fecal samples were collected from the feces donor, the mother (before FMT, and 1, 8, 15, 22, 26, and 50 weeks after FMT), and the infant (meconium at birth and 3 and 6 months after birth). Fecal samples were profiled by deep metagenomic sequencing for strain-level analysis. The microbial transfer was monitored using single nucleotide variants in metagenomes and further compared to a collection of metagenomic samples from 651 healthy infants and 58 healthy adults.
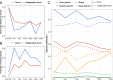
Results: The single FMT procedure led to an uneventful and sustained clinical resolution in the patient, who experienced no further CDI-related symptoms up to 50 weeks after treatment. The gut microbiota of the patient with CDI differed considerably from the healthy donor and was characterized as low in alpha diversity and enriched for several potential pathogens. The FMT successfully normalized the patient's gut microbiota, likely by donor microbiota transfer and engraftment. Importantly, our analysis revealed that some specific strains were transferred from the donor to the patient and then further to the infant, thus demonstrating cross-generational microbial transfer.
Conclusions: The evidence for cross-generational strain transfer following FMT provides novel insights into the dynamics and engraftment of bacterial strains from healthy donors. The data suggests FMT treatment of pregnant women as a potential strategy to introduce beneficial strains or even bacterial consortia to infants, i.e., neonatal seeding. Video Abstract.
Keywords: Clostridioides difficile infection; Engraftment; Fecal microbiota transplantation; Gut microbiota; Infant; Neonatal seeding; Pregnancy; Strain transfer.
© 2022. The Author(s).
Conflict of interest statement
HBN and PNM are employed at Clinical-Microbiomics A/S. The remaining authors declare that they have no competing interests.
Figures



References
-
- Banaei N, Anikst V, Schroeder LF. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2368–2369. - PubMed
-
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–48. - PMC - PubMed
-
- Leffler DA, Lamont JT. Clostridium difficile infection. Longo DL, editor. N Engl J Med. 2015;372:1539–1548. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

