Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications
- PMID: 36352485
- PMCID: PMC9648025
- DOI: 10.1186/s40824-022-00304-3
Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications
Erratum in
-
Correction: Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications.Biomater Res. 2023 Sep 29;27(1):94. doi: 10.1186/s40824-023-00438-y. Biomater Res. 2023. PMID: 37775835 Free PMC article. No abstract available.
Abstract
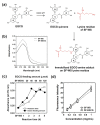
Background: Overproduction of reactive oxygen species (ROS) is known to delay wound healing by causing oxidative tissue damage and inflammation. The green tea catechin, (-)-Epigallocatechin-3-O-gallate (EGCG), has drawn a great deal of interest due to its strong ROS scavenging and anti-inflammatory activities. In this study, we developed EGCG-grafted silk fibroin hydrogels as a potential wound dressing material.
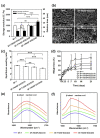
Methods: The introduction of EGCG to water-soluble silk fibroin (SF-WS) was accomplished by the nucleophilic addition reaction between lysine residues in silk proteins and EGCG quinone at mild basic pH. The resulting SF-EGCG conjugate was co-crosslinked with tyramine-substituted SF (SF-T) via horseradish peroxidase (HRP)/H2O2 mediated enzymatic reaction to form SF-T/SF-EGCG hydrogels with series of composition ratios.
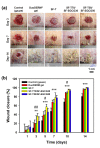
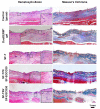
Results: Interestingly, SF-T70/SF-EGCG30 hydrogels exhibited rapid in situ gelation (< 30 s), similar storage modulus to human skin (≈ 1000 Pa) and superior wound healing performance over SF-T hydrogels and a commercial DuoDERM® gel dressings in a rat model of full thickness skin defect.
Conclusion: This study will provide useful insights into a rational design of ROS scavenging biomaterials for wound healing applications.
Keywords: EGCG; Hydrogel; Reactive oxygen species; Silk fibroin; Wound healing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation.Biomaterials. 2020 Feb;232:119720. doi: 10.1016/j.biomaterials.2019.119720. Epub 2019 Dec 23. Biomaterials. 2020. PMID: 31896515 Free PMC article.
-
Fabrication andin vitroevaluation of photo cross-linkable silk fibroin-epsilon-poly-L-lysine hydrogel for wound repair.Biomed Mater. 2023 Aug 22;18(5). doi: 10.1088/1748-605X/acef86. Biomed Mater. 2023. PMID: 37567188
-
EGCG-crosslinked carboxymethyl chitosan-based hydrogels with inherent desired functions for full-thickness skin wound healing.J Mater Chem B. 2022 May 25;10(20):3927-3935. doi: 10.1039/d2tb00074a. J Mater Chem B. 2022. PMID: 35485772
-
Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review.Molecules. 2021 Oct 11;26(20):6123. doi: 10.3390/molecules26206123. Molecules. 2021. PMID: 34684703 Free PMC article. Review.
-
Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels.Acta Biomater. 2021 Apr 15;125:57-71. doi: 10.1016/j.actbio.2021.02.018. Epub 2021 Feb 16. Acta Biomater. 2021. PMID: 33601067 Review.
Cited by
-
Greener healing: sustainable nanotechnology for advanced wound care.Discov Nano. 2024 Aug 13;19(1):127. doi: 10.1186/s11671-024-04061-1. Discov Nano. 2024. PMID: 39136798 Free PMC article. Review.
-
The wound healing effect of botanicals and pure natural substances used in in vivo models.Inflammopharmacology. 2023 Apr;31(2):755-772. doi: 10.1007/s10787-023-01157-5. Epub 2023 Feb 22. Inflammopharmacology. 2023. PMID: 36811778 Free PMC article. Review.
-
Polyphenols in wound healing: unlocking prospects with clinical applications.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar;398(3):2459-2485. doi: 10.1007/s00210-024-03538-1. Epub 2024 Oct 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39453503 Review.
-
ROS-scavenging materials for skin wound healing: advancements and applications.Front Bioeng Biotechnol. 2023 Dec 12;11:1304835. doi: 10.3389/fbioe.2023.1304835. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38149175 Free PMC article. Review.
-
Electronic States of Epigallocatechin-3-Gallate in Water and in 1,2-dipalmitoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (Sodium Salt) Liposomes.Int J Mol Sci. 2025 Jan 27;26(3):1084. doi: 10.3390/ijms26031084. Int J Mol Sci. 2025. PMID: 39940852 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

