Reduced grid-like theta modulation in schizophrenia
- PMID: 36352511
- PMCID: PMC10151182
- DOI: 10.1093/brain/awac416
Reduced grid-like theta modulation in schizophrenia
Abstract
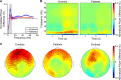
The hippocampal formation has been implicated in the pathophysiology of schizophrenia, with patients showing impairments in spatial and relational cognition, structural changes in entorhinal cortex and reduced theta coherence with medial prefrontal cortex. Both the entorhinal cortex and medial prefrontal cortex exhibit a 6-fold (or 'hexadirectional') modulation of neural activity during virtual navigation that is indicative of grid cell populations and associated with accurate spatial navigation. Here, we examined whether these grid-like patterns are disrupted in schizophrenia. We asked 17 participants with diagnoses of schizophrenia and 23 controls (matched for age, sex and IQ) to perform a virtual reality spatial navigation task during magnetoencephalography. The control group showed stronger 4-10 Hz theta power during movement onset, as well as hexadirectional modulation of theta band oscillatory activity in the right entorhinal cortex whose directional stability across trials correlated with navigational accuracy. This hexadirectional modulation was absent in schizophrenia patients, with a significant difference between groups. These results suggest that impairments in spatial and relational cognition associated with schizophrenia may arise from disrupted grid firing patterns in entorhinal cortex.
Keywords: entorhinal cortex; grid cells; schizophrenia; spatial memory.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- American Psychiatric Association.. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
-
- Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl). 2004;174:151–162. - PubMed
-
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

