Vinculin transmits high-level integrin tensions that are dispensable for focal adhesion formation
- PMID: 36352785
- PMCID: PMC9822790
- DOI: 10.1016/j.bpj.2022.11.013
Vinculin transmits high-level integrin tensions that are dispensable for focal adhesion formation
Abstract
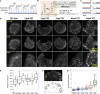
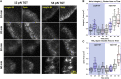
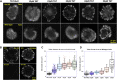
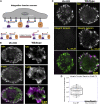
Focal adhesions (FAs) transmit force and mediate mechanotransduction between cells and the matrix. Previous studies revealed that integrin-transmitted force is critical to regulate FA formation. As vinculin is a prominent FA protein implicated in integrin tension transmission, this work studies the relation among integrin tensions (force), vinculin (protein), and FA formation (structure) by integrin tension manipulation, force visualization and vinculin knockout (KO). Two DNA-based integrin tension tools are adopted: tension gauge tether (TGT) and integrative tension sensor (ITS), with TGT restricting integrin tensions under a designed Ttol (tension tolerance) value and ITS visualizing integrin tensions above the Ttol value by fluorescence. Results show that large FAs (area >1 μm2) were formed on the TGT surface with Ttol of 54 pN but not on those with lower Ttol values. Time-series analysis of FA formation shows that focal complexes (area <0.5 μm2) appeared on all TGT surfaces 20 min after cell plating, but only matured to large FAs on TGT with Ttol of 54 pN. Next, we tested FA formation in vinculin KO cells on TGT surfaces. Surprisingly, the Ttol value of TGT required for large FA formation is drastically decreased to 23 pN. To explore the cause, we visualized integrin tensions in both wild-type and vinculin KO cells using ITS. The results showed that integrin tensions in FAs of wild-type cells frequently activate ITS with Ttol of 54 pN. With vinculin KO, however, integrin tensions in FAs became lower and unable to activate 54 pN ITS. Force signal intensities of integrin tensions reported by 33 and 43 pN ITS were also significantly reduced with vinculin KO, suggesting that vinculin is essential to transmit high-level integrin tensions and involved in transmitting intermediate-level integrin tensions in FAs. However, the high-level integrin tensions transmitted by vinculin are not required by FA formation.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Similar articles
-
Integrin molecular tension required for focal adhesion maturation and YAP nuclear translocation.Biochem Biophys Rep. 2022 May 31;31:101287. doi: 10.1016/j.bbrep.2022.101287. eCollection 2022 Sep. Biochem Biophys Rep. 2022. PMID: 35669986 Free PMC article.
-
Integrins outside focal adhesions transmit tensions during stable cell adhesion.Sci Rep. 2016 Nov 15;6:36959. doi: 10.1038/srep36959. Sci Rep. 2016. PMID: 27845380 Free PMC article.
-
Develop Tandem Tension Sensor to Gauge Integrin-Transmitted Molecular Forces.ACS Sens. 2024 Jul 26;9(7):3660-3670. doi: 10.1021/acssensors.4c00756. Epub 2024 Jul 5. ACS Sens. 2024. PMID: 38968930 Free PMC article.
-
Vinculin in cell-cell and cell-matrix adhesions.Cell Mol Life Sci. 2017 Aug;74(16):2999-3009. doi: 10.1007/s00018-017-2511-3. Epub 2017 Apr 11. Cell Mol Life Sci. 2017. PMID: 28401269 Free PMC article. Review.
-
Vinculin-p130Cas interaction is critical for focal adhesion dynamics and mechano-transduction.Cell Biol Int. 2014 Mar;38(3):283-6. doi: 10.1002/cbin.10204. Epub 2013 Nov 27. Cell Biol Int. 2014. PMID: 24497348 Review.
Cited by
-
Hydrogel-based molecular tension fluorescence microscopy for investigating receptor-mediated rigidity sensing.Nat Methods. 2023 Nov;20(11):1780-1789. doi: 10.1038/s41592-023-02037-0. Epub 2023 Oct 5. Nat Methods. 2023. PMID: 37798478
-
Vinculin is essential for sustaining normal levels of endogenous forces at cell-cell contacts.Biophys J. 2023 Dec 5;122(23):4518-4527. doi: 10.1016/j.bpj.2023.10.029. Epub 2023 Oct 29. Biophys J. 2023. PMID: 38350000 Free PMC article.
-
Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: insights into adhesomes, mechanical forces, and signaling pathways.Front Cell Dev Biol. 2023 Nov 30;11:1221306. doi: 10.3389/fcell.2023.1221306. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099295 Free PMC article. Review.
-
A Novel and Versatile Microfluidic Device for Cell Assays under Radio Frequency Exposure.Biosensors (Basel). 2023 Jul 27;13(8):763. doi: 10.3390/bios13080763. Biosensors (Basel). 2023. PMID: 37622849 Free PMC article.
-
Live-cell imaging of single integrin tensions with minimal background fluorescence noise.Biophys J. 2025 Apr 1;124(7):1085-1094. doi: 10.1016/j.bpj.2025.02.002. Epub 2025 Feb 10. Biophys J. 2025. PMID: 39935179
References
-
- Geiger B., Bershadsky A., et al. Yamada K.M. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. - PubMed
-
- Evans E.A., Calderwood D.A. Forces and bond dynamics in cell adhesion. Science. 2007;316:1148–1153. - PubMed
-
- Friedland J.C., Lee M.H., Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

