Efficacy of Asymmetric siRNA Targeting Androgen Receptors for the Treatment of Androgenetic Alopecia
- PMID: 36352823
- PMCID: PMC9812025
- DOI: 10.1021/acs.molpharmaceut.2c00510
Efficacy of Asymmetric siRNA Targeting Androgen Receptors for the Treatment of Androgenetic Alopecia
Abstract
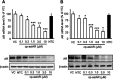
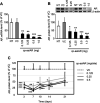
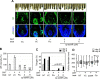
Asymmetric small interfering RNAs (asiRNAs) that mediate RNA interference have been investigated for therapeutic use in various tissues, including skin tissue. Androgenetic alopecia (AGA) is caused by a combination of genetic factors, resulting in sensitivity to dihydrotestosterone (DHT), which binds to the androgen receptor (AR) to mediate a series of biomolecular changes leading to hair loss. This study aimed to evaluate the therapeutic potential of a cell-penetrating, AR-targeting asiRNA (cp-asiAR) for AGA treatment, which was designed to silence the AR gene. AGA mouse models were developed by stimulation with DHT, and ex vivo human scalp tissues were also used for analysis. Cp-asiAR-mediated changes in mRNA expression and protein levels of AR were assessed along with the examination of phenotypic improvements in mouse model of AGA. We also assessed downstream signaling associated with AR in primary human dermal papilla (DP) cells. Several cp-asiARs were screened for selecting the optimal sequence of AR using cell lines in vitro. A cholesterol-conjugated, chemically modified cp-asiAR candidate was optimized under passive uptake conditions in vitro. Intradermal cp-asiAR injection efficiently reduced mRNA and protein levels corresponding to AR in mouse models. Moreover, cp-asiAR injection promoted hair growth in mouse models with DHT-induced AGA. In ex vivo human hair follicle culture, the proportion of telogen hair decreased, and the mean hair bulb diameter increased in the cp-asiAR-treated group. In isolated primary human DP cells, AR expression was effectively downregulated by cp-asiAR. Furthermore, cp-asiAR attenuated DHT-mediated increases in interleukin-6, transforming growth factor-β1, and dickkopf-1 levels. No significant toxicity was observed in DP cells after cp-asiAR treatment. Cp-asiAR treatment showed effective downregulation of AR expression and prevention of DHT-mediated alterations in the hair cycle and hair diameter, indicating its potential as a novel therapeutic option for AGA.
Keywords: androgen receptor; androgenetic alopecia; dermal papilla; dihydrotestosterone; hair follicle; siRNA.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
MitoQ enhances CYP19A1 expression to stimulate WNT/β-catenin signaling pathway for promoting hair growth in androgenetic alopecia.Eur J Pharmacol. 2024 Dec 15;985:177094. doi: 10.1016/j.ejphar.2024.177094. Epub 2024 Nov 14. Eur J Pharmacol. 2024. PMID: 39547405
-
A mouse model of androgenetic alopecia.Endocrinology. 2010 May;151(5):2373-80. doi: 10.1210/en.2009-1474. Epub 2010 Mar 16. Endocrinology. 2010. PMID: 20233794
-
Weekly treatment with SAMiRNA targeting the androgen receptor ameliorates androgenetic alopecia.Sci Rep. 2022 Jan 31;12(1):1607. doi: 10.1038/s41598-022-05544-w. Sci Rep. 2022. PMID: 35102171 Free PMC article. Clinical Trial.
-
Molecular mechanisms of androgenetic alopecia.Exp Gerontol. 2002 Aug-Sep;37(8-9):981-90. doi: 10.1016/s0531-5565(02)00093-1. Exp Gerontol. 2002. PMID: 12213548 Review.
-
Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia.Arch Dermatol Res. 2018 Jul;310(5):391-399. doi: 10.1007/s00403-018-1826-8. Epub 2018 Mar 16. Arch Dermatol Res. 2018. PMID: 29549490 Review.
Cited by
-
Recent approaches of antibody therapeutics in androgenetic alopecia.Front Pharmacol. 2024 Aug 16;15:1434961. doi: 10.3389/fphar.2024.1434961. eCollection 2024. Front Pharmacol. 2024. PMID: 39221145 Free PMC article. Review.
-
Jiawei Erzhiwan Ameliorates Androgenetic Alopecia by Regulating the SIRT1/JNK/p38 MAPK Pathway.Drug Des Devel Ther. 2025 Apr 1;19:2393-2409. doi: 10.2147/DDDT.S501823. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40190813 Free PMC article.
-
Delivery Strategies of siRNA Therapeutics for Hair Loss Therapy.Int J Mol Sci. 2024 Jul 11;25(14):7612. doi: 10.3390/ijms25147612. Int J Mol Sci. 2024. PMID: 39062852 Free PMC article. Review.
-
Curcumin-primed milk-derived extracellular vesicles remodel hair follicle microenvironment for the treatment of androgenetic alopecia.Regen Biomater. 2025 May 30;12:rbaf051. doi: 10.1093/rb/rbaf051. eCollection 2025. Regen Biomater. 2025. PMID: 40735523 Free PMC article.
-
Effects of oxytocin receptor agonists on hair growth promotion.Sci Rep. 2024 Oct 13;14(1):23935. doi: 10.1038/s41598-024-74962-9. Sci Rep. 2024. PMID: 39397061 Free PMC article.
References
-
- Dorsett Y.; Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discov. 2004, 3, 318–329. 10.1038/nrd1345. - DOI - PubMed
- Reich S. J.; Fosnot J.; Kuroki A.; Tang W.; Yang X.; Maguire A. M.; Bennett J.; Tolentino M. J. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol. Vis. 2003, 9, 210–216. - PubMed
- Hwang J.; Chang C.; Kim J. H.; Oh C. T.; Lee H. N.; Lee C.; Oh D.; Lee C.; Kim B.; Hong S. W.; Lee D. K. Development of Cell-Penetrating Asymmetric Interfering RNA Targeting Connective Tissue Growth Factor. J. Invest. Dermatol. 2016, 136, 2305–2313. 10.1016/j.jid.2016.06.626. - DOI - PubMed
-
- Chang C. I.; Yoo J. W.; Hong S. W.; Lee S. E.; Kang H. S.; Sun X.; Rogoff H. A.; Ban C.; Kim S.; Li C. J.; Lee D. K. Asymmetric shorter-duplex siRNA structures trigger efficient gene silencing with reduced nonspecific effects. Mol. Ther. 2009, 17, 725–732. 10.1038/mt.2008.298. - DOI - PMC - PubMed
- Hong S. W.; Park J. H.; Yun S.; Lee C. H.; Shin C.; Lee D. K. Effect of the guide strand 3’-end structure on the gene-silencing potency of asymmetric siRNA. Biochem. J. 2014, 461, 427–434. 10.1042/bj20140407. - DOI - PubMed
-
- Oh J. W.; Kloepper J.; Langan E. A.; Kim Y.; Yeo J.; Kim M. J.; Hsi T.-C.; Rose C.; Yoon G. S.; Lee S.-J.; Seykora J.; Kim J. C.; Sung Y. K.; Kim M.; Paus R.; Plikus M. V. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Invest. Dermatol. 2016, 136, 34–44. 10.1038/JID.2015.354. - DOI - PMC - PubMed
-
- Lee M. Y.; Wang H. Z.; White T. W.; Brooks T.; Pittman A.; Halai H.; Petrova A.; Xu D.; Hart S. L.; Kinsler V. A.; di W. L. Allele-Specific Small Interfering RNA Corrects Aberrant Cellular Phenotype in Keratitis-Ichthyosis-Deafness Syndrome Keratinocytes. J. Invest. Dermatol. 2020, 140, 1035–1044.e7. 10.1016/j.jid.2019.09.022. - DOI - PMC - PubMed
- Sun B. K.; Tsao H. Small RNAs in development and disease. J. Am. Acad. Dermatol. 2008, 59, 725–737. 10.1016/j.jaad.2008.08.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

