Benralizumab monotherapy was insufficient to induce remission in patients with active eosinophilic granulomatosis with polyangiitis
- PMID: 36353062
- PMCID: PMC9637965
- DOI: 10.1016/j.rmcr.2022.101763
Benralizumab monotherapy was insufficient to induce remission in patients with active eosinophilic granulomatosis with polyangiitis
Abstract
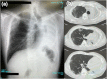
Eosinophils play an important pathogenetic role in the development of eosinophilic granulomatosis with polyangiitis (EGPA). EGPA has long been treated with systemic corticosteroids and immunosuppressive agents. However, in recent years, biologic agents targeting eosinophils (anti-IL-5 antibody; mepolizumab) have also been used. Evidence regarding the effectiveness of using benralizumab, anti-IL-5 receptor α monoclonal antibody that depletes eosinophils via antibody-dependent cell-mediated cytotoxicity, has been growing. Benralizumab is used as a steroid-sparing treatment option for EGPA. Clinical studies have evaluated the effects of using mepolizumab or benralizumab in combination with steroids for the treatment of EGPA. However, to date, there have been no reports of using biologics alone. Herein, we describe the case of a patient with active EGPA refractory to benralizumab monotherapy. The patient achieved significant improvement in symptoms after administration of corticosteroids during hospitalization. Benralizumab monotherapy might not be considered a therapeutic option for patients with active EGPA in whom corticosteroids are initially indicated.
Keywords: ACR/EULAR, American College Rheumatology (ACR)/ European Alliance of Associations for Rheumatology (EULAR); Benralizumab; CT, computed tomography; Corticosteroid; EGPA, eosinophilic granulomatosis with polyangiitis; Eosinophilic granulomatosis with polyangiitis; IL, interleukin; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibody.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Gokhale M., Bell C.F., Doyle S., et al. Prevalence of eosinophilic granulomatosis with polyangitis and associated health care utilization among patients with concomitant asthma in US commercial claims database. J. Clin. Rheumatol. 2019;27:107–113. doi: 10.1097/rhu.0000000000001198. - DOI - PMC - PubMed
-
- FitzGerald J.M., Bleecker E.R., Nair P., Korn S., Ohta K., Lommatzsch M., Ferguson G.T., Busse W.W., Barker P., Sproule S., Gilmartin G., Werkström V., Aurivillius M., Goldman M. CALIMA study investigators, Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

