Characterization of autonomic symptom burden in long COVID: A global survey of 2,314 adults
- PMID: 36353127
- PMCID: PMC9639503
- DOI: 10.3389/fneur.2022.1012668
Characterization of autonomic symptom burden in long COVID: A global survey of 2,314 adults
Abstract
Background: Autonomic dysfunction is a known complication of post-acute sequelae of SARS-CoV-2 (PASC)/long COVID, however prevalence and severity are unknown.
Objective: To assess the frequency, severity, and risk factors of autonomic dysfunction in PASC, and to determine whether severity of acute SARS-CoV-2 infection is associated with severity of autonomic dysfunction.
Design: Cross-sectional online survey of adults with PASC recruited through long COVID support groups between October 2020 and August 2021.
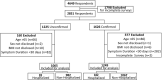
Participants: 2,413 adults ages 18-64 years with PASC including patients who had a confirmed positive test for COVID-19 (test-confirmed) and participants who were diagnosed with COVID-19 based on clinical symptoms alone.
Main measures: The main outcome measure was the Composite Autonomic Symptom 31 (COMPASS-31) total score, used to assess global autonomic dysfunction. Test-confirmed hospitalized vs. test-confirmed non-hospitalized participants were compared to determine if the severity of acute SARS-CoV-2 infection was associated with the severity autonomic dysfunction.
Key results: Sixty-six percent of PASC patients had a COMPASS-31 score >20, suggestive of moderate to severe autonomic dysfunction. COMPASS-31 scores did not differ between test-confirmed hospitalized and test-confirmed non-hospitalized participants [28.95 (15.62, 46.60) vs. 26.4 (13.75, 42.10); p = 0.06].
Conclusions: Evidence of moderate to severe autonomic dysfunction was seen in 66% of PASC patients in our study, independent of hospitalization status, suggesting that autonomic dysfunction is highly prevalent in the PASC population and independent of the severity of acute COVID-19 illness.
Keywords: autonomic; autonomic dysfunction; dysautonomia; long COVID; post-acute COVID; post-acute sequelae of COVID-19.
Copyright © 2022 Larsen, Stiles, Shaik, Schneider, Muppidi, Tsui, Geng, Bonilla and Miglis.
Conflict of interest statement
Author NL—Grant from Dysautonomia International; Stock or stock options in Health Care Select SPDR, Ishares Biotechnology. Author LS—Consulting fees from Eisai Pharmaceuticals (ad board, unrelated to current manuscript) and Jazz Pharmaceuticals (ad board, unrelated to current manuscript); Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Jazz Pharmaceuticals, Xywav, Xyrem and Sunosi; Payment for expert testimony from City and County of San Francisco (sleep deprivation cases); Support for attending meetings and/or travel from Jazz Pharmaceuticals (Psych Congress 2021, poster presentation); Leadership or fiduciary role in other board, society, committee or advocacy group from Graduate Education Subcommittee Chair (AAN, unpaid), Graduate Education Subcommittee Chair (AAN, unpaid), Editorial Board (Practical Neurology, unpaid); Stock or stock options in Alphabet. Author SM—Grants or contracts from the Myasthenia Gravis Foundation of America; Consulting fees from Argenx, Alexion and UCB pharma (consultation and advisory board meetings). Author LG—Consulting fees from United Health Group. Author MM—Royalties from Elsvier Inc.; Consulting fees from 2nd MD, Infinite MD and Guidepoint LLC; Payments for CME lectures from MED-IQ; Payment for expert testimony from Van Cott Talamante (expert witness); Medical Advisory Board of Dysautonomia International (unpaid), and MSA Coalitionn (unpaid). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

