An in vivo assay to study locomotion in Caenorhabditis elegans
- PMID: 36353356
- PMCID: PMC9637769
- DOI: 10.1016/j.mex.2022.101890
An in vivo assay to study locomotion in Caenorhabditis elegans
Abstract
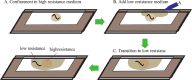
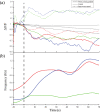
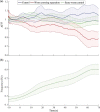
Adaptation in the sensory-mechanical loop during locomotion is a powerful mechanism that allows organisms to survive in different conditions and environments. Motile animals need to alter motion patterns in different environments. For example, crocodiles and other animals can walk on solid ground but switch to swimming in water beds. The nematode Caenorhabditis elegans also shows adaptability by employing thrashing behaviour in low viscosity media and crawling in high viscosity media. The mechanism that enables this adaptability is an active area of research. It has been attributed previously to neuro-modulation by dopamine and serotonin. This study introduces an experimental assay to physiologically investigate the neuronal mechanisms of modulation of locomotion by dopamine. The technique is utilized to test gait switching while imaging the mechanosensory dopaminergic neurons PDE. Results revealed their role to be not limited to touch sensation, but to sensing surrounding environment resistance as well. The significance of such characterization is improving our understanding of dopamine gait switching which gets impaired in Parkinson's disease.-A locomotion pattern switching system was devised to allow studying this process in vivo in the nematode C. elegans.-This system allowed the study of dopaminergic neurons PDE response as the worms switched from crawling to swimming.
Keywords: Behavioral assay; Locomotion; Neuromodulation.
© 2022 The Author.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Cordelieres F.P. Institut Curie; Orsay (France): 2005. Manual Tracking.
-
- Maru M., Igarashi Y., Arai S., Hashimoto K. System Integration (SII), 2010 IEEE/SICE International Symposium on. IEEE; 2010. Fluorescent microscope system to track a particular region of C. elegans; pp. 347–352.
LinkOut - more resources
Full Text Sources

