Molecular mechanisms of plant growth promotion for methylotrophic Bacillus aryabhattai LAD
- PMID: 36353455
- PMCID: PMC9637944
- DOI: 10.3389/fmicb.2022.917382
Molecular mechanisms of plant growth promotion for methylotrophic Bacillus aryabhattai LAD
Abstract
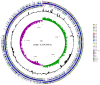
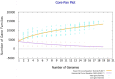
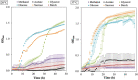
Plant growth-promoting rhizobacteria (PGPR) can produce hormone-like substances, promote plant nutrient uptake, enhance plant resistance, inhibit the growth of pathogenic bacteria, and induce plant resistance to biotic and abiotic stresses. Bacillus is one of the most studied genera that promote plant root development. Since its discovery in 2009, B. aryabhattai has shown promising properties such as promoting plant growth and improving crop yield. However, the mechanisms of B. aryabhattai promoting plant growth remain to be investigated. In this study, the chromosome of B. aryabhattai strain LAD and five plasmids within the cell were sequenced and annotated. The genome, with a length of 5,194,589 bp and 38.12% GC content, contains 5,288 putative protein-coding genes, 39 rRNA, and 112 tRNA. The length of the five plasmids ranged from 116,519 to 212,484 bp, and a total of 810 putative protein-coding genes, 4 rRNA, and 32 tRNA were predicted in the plasmids. Functional annotation of the predicted genes revealed numerous genes associated with indole-3 acetic acid (IAA) and exopolysaccharides (EPSs) biosynthesis, membrane transport, nitrogen cycle metabolism, signal transduction, cell mobility, stress response, and antibiotic resistance on the genome which benefits the plants. Genes of carbohydrate-active enzymes were detected in both the genome and plasmids suggesting that LAD has the capacity of synthesizing saccharides and utilizing organic materials like root exudates. LAD can utilize different carbon sources of varied carbon chain length, i.e., methanol, acetate, glycerol, glucose, sucrose, and starch for growth and temperature adaptation suggesting a high versatility of LAD for thriving in fluctuating environments. LAD produced the most EPSs with sucrose as sole carbon source, and high concentration of IAA was produced when the maize plant was cultivated with LAD, which may enhance plant growth. LAD significantly stimulated the development of the maize root. The genome-based information and experimental evidence demonstrated that LAD with diverse metabolic capabilities and positive interactions with plants has tremendous potential for adaptation to the dynamic soil environments and promoting plant growth.
Keywords: carbohydrate-active enzymes; growth promoting; indole acetic acid; rhizosphere; sequencing.
Copyright © 2022 Deng, Liang, Zhang, Li, Wang and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Mechanisms of ROS-mediated interactions between Bacillus aryabhattai LAD and maize roots to promote plant growth.BMC Microbiol. 2024 Sep 6;24(1):327. doi: 10.1186/s12866-024-03479-y. BMC Microbiol. 2024. PMID: 39242527 Free PMC article.
-
Genome-Guided Insights into the Plant Growth Promotion Capabilities of the Physiologically Versatile Bacillus aryabhattai Strain AB211.Front Microbiol. 2017 Mar 21;8:411. doi: 10.3389/fmicb.2017.00411. eCollection 2017. Front Microbiol. 2017. PMID: 28377746 Free PMC article.
-
Bacillus aryabhattai LAD impacts rhizosphere bacterial community structure and promotes maize plant growth.J Sci Food Agric. 2022 Nov;102(14):6650-6657. doi: 10.1002/jsfa.12032. Epub 2022 Jun 10. J Sci Food Agric. 2022. PMID: 35603593
-
Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress.Saudi J Biol Sci. 2019 Sep;26(6):1291-1297. doi: 10.1016/j.sjbs.2019.05.004. Epub 2019 May 20. Saudi J Biol Sci. 2019. PMID: 31516360 Free PMC article. Review.
-
The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments.Biotechnol Adv. 2014 Mar-Apr;32(2):429-48. doi: 10.1016/j.biotechadv.2013.12.005. Epub 2013 Dec 28. Biotechnol Adv. 2014. PMID: 24380797 Review.
Cited by
-
Biocontrol and Growth Promotion Potential of Bacillus subtilis CTXW 7-6-2 against Rhizoctonia solani that Causes Tobacco Target Spot Disease.Pol J Microbiol. 2024 Mar 4;73(1):29-38. doi: 10.33073/pjm-2024-004. eCollection 2024 Mar 1. Pol J Microbiol. 2024. PMID: 38437465 Free PMC article.
-
Mechanisms of ROS-mediated interactions between Bacillus aryabhattai LAD and maize roots to promote plant growth.BMC Microbiol. 2024 Sep 6;24(1):327. doi: 10.1186/s12866-024-03479-y. BMC Microbiol. 2024. PMID: 39242527 Free PMC article.
-
Functional and genomic analyses of plant growth promoting traits in Priestia aryabhattai and Paenibacillus sp. isolates from tomato rhizosphere.Sci Rep. 2025 Jan 28;15(1):3498. doi: 10.1038/s41598-025-87390-0. Sci Rep. 2025. PMID: 39875501 Free PMC article.
-
Plant Growth-Promoting Rhizobacteria Enhance Sweet Cherry Root System Development Through the Production of Volatile Organic Compounds.Foods. 2025 Jul 3;14(13):2369. doi: 10.3390/foods14132369. Foods. 2025. PMID: 40647121 Free PMC article.
-
Novel Bacillus and Prestia isolates from Dwarf century plant enhance crop yield and salinity tolerance.Sci Rep. 2024 Jun 25;14(1):14645. doi: 10.1038/s41598-024-65632-x. Sci Rep. 2024. PMID: 38918548 Free PMC article.
References
-
- Backer R., Rokem J. S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., et al. . (2018). Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 9:1473. doi: 10.3389/fpls.2018.01473, PMID: - DOI - PMC - PubMed
-
- Berta B. (2000). Putative sites for nutrient uptake in arbuscular mycorrhizal fungi. Plant Soil 226, 263–274. doi: 10.1023/A:1026456818903 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

