Chaigui granule exerts anti-depressant effects by regulating the synthesis of Estradiol and the downstream of CYP19A1-E2-ERKs signaling pathway in CUMS-induced depressed rats
- PMID: 36353500
- PMCID: PMC9637986
- DOI: 10.3389/fphar.2022.1005438
Chaigui granule exerts anti-depressant effects by regulating the synthesis of Estradiol and the downstream of CYP19A1-E2-ERKs signaling pathway in CUMS-induced depressed rats
Abstract
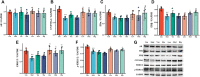
Background: There is a significant gender difference in the prevalence of depression. Recent studies have shown that estrogen plays a crucial role in depression. Therefore, studying the specific mechanism of estrogen's role in depression can provide new ideas to address the treatment of depression. Chaigui granule has been shown to have exact antidepressant efficacy, and the contents of saikosaponin (a, b1, b2, d) and paeoniflorin in Chaigui granule are about 0.737% and 0.641%, respectively. Some studies have found that they can improve depression-induced decrease in testosterone (T) levels (∼36.99% decrease compared to control). However, whether Chaigui granule can exert antidepressant efficacy by regulating estrogen is still unclear. This study aimed to elucidate the regulation of estrogen levels by Chaigui granule and the underlying mechanism of its anti-depressant effect. Methods: Eighty-four male Sprague-Dawley (SD) rats were modeled using a chronic unpredictable mild stress (CUMS) procedure. The administration method was traditional oral gavage administration, and behavioral indicators were used to evaluate the anti-depressant effect of Chaigui granule. Enzyme-linked immunosorbent assay (ELISA) was adopted to assess the modulating impact of Chaigui granule on sex hormones. Then, reverse transcription-quantitative PCR (RT-qPCR), and Western blot (WB) techniques were employed to detect extracellular regulated protein kinases (ERK) signaling-related molecules downstream of estradiol in the hippocampus tissue. Results: The administration of Chaigui granule significantly alleviated the desperate behavior of CUMS-induced depressed rats. According to the results, we found that Chaigui granule could upregulate the level of estradiol (E2) in the serum (∼46.56% increase compared to model) and hippocampus (∼26.03% increase compared to model) of CUMS rats and increase the levels of CYP19A1 gene and protein, which was the key enzyme regulating the synthesis of T into E2 in the hippocampus. Chaigui granule was also found to have a significant back-regulatory effect on the gene and protein levels of ERβ, ERK1, and ERK2. Conclusion: Chaigui granule can increase the synthesis of E2 in the hippocampus of CUMS-induced depressed rats and further exert antidepressant effects by activating the CYP19A1-E2-ERKs signaling pathway.
Keywords: CYP19A1-E2-ERKs signaling pathway; chaigui granule; chronic unpredictable mild stress; depression; xiaoyao san.
Copyright © 2022 Tian, Qin, Xu, Gao, Zhou, Gao, Qin and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aizawa N., Gandaglia G., Hedlund P., Fujimura T., Fukuhara H., Montorsi F., et al. (2016). URB937, a peripherally restricted inhibitor for fatty acid amide hydrolase, reduces prostaglandin E2 -induced bladder overactivity and hyperactivity of bladder mechano-afferent nerve fibres in rats. BJU Int. 117, 821–828. 10.1111/bju.13223 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

