Neoadjuvant immunotherapy combined with chemotherapy significantly improved patients' overall survival when compared with neoadjuvant chemotherapy in non-small cell lung cancer: A cohort study
- PMID: 36353552
- PMCID: PMC9637677
- DOI: 10.3389/fonc.2022.1022123
Neoadjuvant immunotherapy combined with chemotherapy significantly improved patients' overall survival when compared with neoadjuvant chemotherapy in non-small cell lung cancer: A cohort study
Abstract
Background: Programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors displayed considerable advantages in neoadjuvant therapy of non-small cell lung cancer (NSCLC), but the specific application of neoadjuvant immunotherapy has not been well determined, and the long-term prognostic data of neoadjuvant immunochemotherapy combined with surgical resection of NSCLC remains limited. In this study, we intended to assess the efficacy of the neoadjuvant therapy of the PD-1 inhibitor and long-term prognosis in patients with resectable NSCLC.
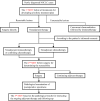
Methods: We retrospectively analyzed NSCLC surgical patients treated with neoadjuvant therapy in our hospital, and divided them into a neoadjuvant chemotherapy group and a neoadjuvant immunotherapy combined with chemotherapy group. The propensity score matching method was used to evaluate the effectiveness of immunotherapy combined with chemotherapy in the treatment of resectable lung cancer, and the long-term prognosis of these two groups was compared.
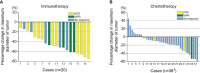
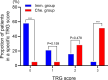
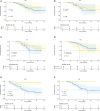
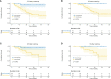
Results: A total of 62 cases were enrolled, including 20 patients (20/62, 32.26%) in the immunotherapy group and 42 patients (42/62, 67.74%) in the chemotherapy group. The clinical baseline data of these two groups were balanced. In the immunotherapy group, all patients had tumor regression in imaging finding (tumor regression ratio: 11.88% - 75.00%). In the chemotherapy group, 30 patients had tumor regression (tumor regression ratio: 2.70% - 58.97%). The R0 removal rates of cancers were comparable between the immunotherapy group and chemotherapy group (19/20, 95.00% vs. 39/42, 92.86%, P=1.000). The two groups were balanced in complete minimally invasive surgery, pneumonectomy, operative duration, blood loss, postoperative complications, and hospital stay. The immunotherapy group had more sleeve resection (36.84% vs. 10.26%, p=0.039) including bronchial sleeve and vascular sleeve, higher pathological complete response (pCR) rate (57.89% vs. 5.13%, P<0.001) and major pathologic response (MPR) rate (78.95% vs. 10.26%, P<0.001). There were no differences in survival curves for: smoker and non-smoker, squamous cell carcinoma and adenocarcinoma, or right lung cancer and left lung cancer. Moreover, patients who achieved MPR (including pCR) had significantly better overall survival (OS) and disease-free survival (DFS). Patients in immunotherapy group had significantly better OS and longer DFS than those in chemotherapy group.
Conclusions: In conclusion, neoadjuvant immunotherapy combined with chemotherapy can provide better OS and DFS and improving pCR and MPR rates by shrinking tumors.This study has been registered in the Chinese Clinical Trial Registry, number ChiCTR2200060433. http://www.chictr.org.cn/edit.aspx?pid=170157&htm=4.
Keywords: lung resection; neoadjuvant chemotherapy; neoadjuvant immunotherapy; non-small cell lung cancer; prognosis.
Copyright © 2022 Dai, Wu, Wang, Li, Wang, Shen, Zhou, Niu, Deng, Tan, Wang and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

