Short-term safety and immunogenicity of inactivated and peptide-based SARS-CoV-2 vaccines in patients with endocrine-related cancer
- PMID: 36353624
- PMCID: PMC9637626
- DOI: 10.3389/fimmu.2022.1028246
Short-term safety and immunogenicity of inactivated and peptide-based SARS-CoV-2 vaccines in patients with endocrine-related cancer
Abstract
Background: The aim of this study was to explore the short-term safety and immunogenicity of inactivated and peptide-based SARS-CoV-2 vaccines in patients with endocrine-related cancer (ER).
Methods: Eighty-eight patients with ER cancer and 82 healthy controls who had completed a full course of inactivated or peptide-based SARS-CoV-2 vaccines were recruited. Adverse events (AEs) were recorded. Responses to receptor-binding domain IgG antibody (anti-RBD-IgG), neutralizing antibodies (NAbs) and RBD+ memory B cells (MBCs) were evaluated.
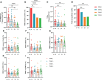
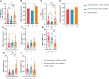
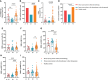
Results: Approximately 26.14% (23/88) of patients with ER cancer reported AEs within 7 days, which was comparable to that reported by healthy controls (24.39%, 20/82). Both the overall seroprevalence of anti-RBD-IgG and NAbs was obviously lower in the cancer group (70.45% vs. 86.59%, P < 0.05; 69.32% vs. 82.93%, P < 0.05, respectively). Anti-RBD-IgG and NAbs titers exhibited similar results, and dropped gradually over time. Patients with ongoing treatment had an attenuated immune response, especially in patients receiving active chemotherapy. The frequency of overall RBD+ MBCs was similar between the two groups, but the percentage of active MBCs was remarkably reduced in patients with ER cancer. Unlike antibody titers, MBCs responses were relatively constant over time.
Conclusion: Inactivated and peptide-based COVID-19 vaccines were well tolerated, but with lower immunogenicity for ER cancer patients. More intensive antibody monitoring and timely booster immunization is recommended for patients with ER cancer presenting disordered subpopulations of RBD+ MBCs.
Keywords: COVID-19; SARS-COV-2; cancer; memory B cells; vaccine.
Copyright © 2022 Song, Liu, Pan, Liu, Tan, Deng, Deng, Lin, Chen, Peng, Ren and Ming.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Safety and immunogenicity of inactivated COVID-19 vaccine in patients with metabolic syndrome: A cross-sectional observational study.Front Public Health. 2022 Dec 23;10:1067342. doi: 10.3389/fpubh.2022.1067342. eCollection 2022. Front Public Health. 2022. PMID: 36620297 Free PMC article.
-
Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people with gastrointestinal cancer.Int J Infect Dis. 2022 Sep;122:874-884. doi: 10.1016/j.ijid.2022.07.050. Epub 2022 Jul 26. Int J Infect Dis. 2022. PMID: 35905950 Free PMC article.
-
Impaired antibody responses were observed in patients with type 2 diabetes mellitus after receiving the inactivated COVID-19 vaccines.Virol J. 2023 Feb 7;20(1):22. doi: 10.1186/s12985-023-01983-7. Virol J. 2023. PMID: 36750902 Free PMC article.
-
Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV.Emerg Microbes Infect. 2022 Dec;11(1):1126-1134. doi: 10.1080/22221751.2022.2059401. Emerg Microbes Infect. 2022. PMID: 35369854 Free PMC article.
-
Immune responses to inactivated COVID-19 vaccine were decreased in Chinese patients with chronic respiratory diseases.Int J Med Sci. 2023 Apr 23;20(6):737-748. doi: 10.7150/ijms.78766. eCollection 2023. Int J Med Sci. 2023. PMID: 37213672 Free PMC article.
References
-
- Taghizadeh-Hesary F, Porouhan P, Soroosh D, Peyroshabany B, Shahidsales S, Keykhosravi B, et al. . Covid-19 in cancer and non-cancer patients. Int J Cancer Manage (2021) 14(4):e110907. doi: 10.5812/ijcm.110907 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

