Unbalanced Arginine pathway and altered maturation of pleural macrophages in Th2-deficient mice during Litomosoides sigmodontis filarial infection
- PMID: 36353644
- PMCID: PMC9637854
- DOI: 10.3389/fimmu.2022.866373
Unbalanced Arginine pathway and altered maturation of pleural macrophages in Th2-deficient mice during Litomosoides sigmodontis filarial infection
Abstract
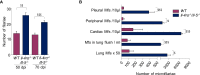
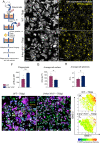
Filarial parasites are tissue dwelling worms transmitted by hematophagous vectors. Understanding the mechanisms regulating microfilariae (the parasite offspring) development is a prerequisite for controlling transmission in filarial infections. Th2 immune responses are key for building efficient anti-parasite responses but have been shown to also lead to detrimental tissue damage in the presence of microfilariae. Litomosoides sigmodontis, a rodent filaria residing in the pleural cavity was therefore used to characterize pleuropulmonary pathology and associated immune responses in wild-type and Th2 deficient mice. Wild-type and Th2-deficient mice (Il-4rα-/-/Il-5-/- ) were infected with L. sigmodontis and parasite outcome was analyzed during the patent phase (when microfilariae are in the general circulation). Pleuropulmonary manifestations were investigated and pleural and bronchoalveolar cells were characterized by RNA analysis, imaging and/or flow cytometry focusing on macrophages. Il-4rα-/-/Il-5-/- mice were hypermicrofilaremic and showed an enhanced filarial survival but also displayed a drastic reduction of microfilaria-driven pleural cavity pathologies. In parallel, pleural macrophages from Il-4rα-/-/Il-5-/- mice lacked expression of prototypical alternative activation markers RELMα and Chil3 and showed an altered balance of some markers of the arginine metabolic pathway. In addition, monocytes-derived F4/80intermediate macrophages from infected Il-4rα-/-/Il-5-/- mice failed to mature into resident F4/80high large macrophages. Altogether these data emphasize that the presence of both microfilariae and IL-4R/IL-5 signaling are critical in the development of the pathology and in the phenotype of macrophages. In Il-4rα-/-/Il-5-/- mice, the balance is in favor of parasite development while limiting the pathology associated with the host immune response.
Keywords: filariasis; lung; macrophage; microfilaria; nematode; parasite; pleura.
Copyright © 2022 Remion, Gal, Chaouch, Rodrigues, Lhermitte-Vallarino, Alonso, Kohl, Hübner, Fercoq and Martin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
IL-4/5 signalling plays an important role during Litomosoides sigmodontis infection, influencing both immune system regulation and tissue pathology in the thoracic cavity.Int J Parasitol. 2017 Dec;47(14):951-960. doi: 10.1016/j.ijpara.2017.06.009. Epub 2017 Aug 30. Int J Parasitol. 2017. PMID: 28859850
-
IL-4 receptor dependent expansion of lung CD169+ macrophages in microfilaria-driven inflammation.PLoS Negl Trop Dis. 2019 Aug 30;13(8):e0007691. doi: 10.1371/journal.pntd.0007691. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31469835 Free PMC article.
-
Susceptibility to L. sigmodontis infection is highest in animals lacking IL-4R/IL-5 compared to single knockouts of IL-4R, IL-5 or eosinophils.Parasit Vectors. 2019 May 20;12(1):248. doi: 10.1186/s13071-019-3502-z. Parasit Vectors. 2019. PMID: 31109364 Free PMC article.
-
The immune response of inbred laboratory mice to Litomosoides sigmodontis: A route to discovery in myeloid cell biology.Parasite Immunol. 2020 Jul;42(7):e12708. doi: 10.1111/pim.12708. Epub 2020 Mar 21. Parasite Immunol. 2020. PMID: 32145033 Free PMC article. Review.
-
Immune responses to filarial infection in laboratory mice.Med Microbiol Immunol. 1997 Mar;185(4):207-15. doi: 10.1007/s004300050032. Med Microbiol Immunol. 1997. PMID: 9138292 Review.
Cited by
-
Host environment shapes filarial parasite fitness and Wolbachia endosymbionts dynamics.PLoS Pathog. 2025 Jul 11;21(7):e1013301. doi: 10.1371/journal.ppat.1013301. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40644522 Free PMC article.
-
How to train your myeloid cells: a way forward for helminth vaccines?Front Immunol. 2023 May 30;14:1163364. doi: 10.3389/fimmu.2023.1163364. eCollection 2023. Front Immunol. 2023. PMID: 37325618 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

