Real-world Treatment Sequencing in Patients with Metastatic Castration-resistant Prostate Cancer: Results from the Prospective, International, Observational Prostate Cancer Registry
- PMID: 36353661
- PMCID: PMC9637537
- DOI: 10.1016/j.euros.2022.08.018
Real-world Treatment Sequencing in Patients with Metastatic Castration-resistant Prostate Cancer: Results from the Prospective, International, Observational Prostate Cancer Registry
Abstract
Background: Prostate cancer has a multifaceted treatment pattern. Evidence is lacking for optimal treatment sequences for metastatic castration-resistant prostate cancer (mCRPC).
Objective: To increase the understanding of real-world treatment pathways and outcomes in patients with mCRPC.
Design setting and participants: A prospective, noninterventional, real-world analysis of 3003 patients with mCRPC in the Prostate Cancer Registry (PCR; NCT02236637) from June 14, 2013 to July 9, 2018 was conducted.
Intervention: Patients received first- and second-line hormonal treatment and chemotherapy as follows: abiraterone acetate plus prednisone (abiraterone)-docetaxel (ABI-DOCE), abiraterone-enzalutamide (ABI-ENZA), abiraterone-radium-223 (ABI-RAD), docetaxel-abiraterone (DOCE-ABI), docetaxel-cabazitaxel (DOCE-CABA), docetaxel-enzalutamide (DOCE-ENZA), and enzalutamide-docetaxel (ENZA-DOCE).
Outcome measurements and statistical analysis: Baseline patient characteristics, quality of life, mCRPC treatments, and efficacy outcomes (progression and survival) were presented descriptively.
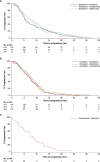
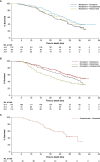
Results and limitations: Data from 727 patients were eligible for the analysis (ABI-DOCE n = 178, ABI-ENZA n = 99, ABI-RAD n = 27, DOCE-ABI n = 191, DOCE-CABA n = 74, DOCE-ENZA n = 116, and ENZA-DOCE n = 42). Demographics and disease characteristics among patients between different sequences varied greatly. Most patients who started on abiraterone or enzalutamide stopped therapy because of disease progression. No randomisation to allow treatment/sequence comparisons limited this observational study.
Conclusions: The real-world PCR data complement clinical trial data, reflecting more highly selected patient populations than seen in routine clinical practice. Baseline characteristics play a role in mCRPC first-line treatment selection, but other factors, such as treatment availability, have an impact. Efficacy observations are limited and should be interpreted with caution.
Patient summary: Baseline characteristics appear to have a role in the first-line treatment selection of metastatic castration-resistant prostate cancer in the real-world setting. First-line abiraterone acetate plus prednisone seems to be the preferred treatment option for older patients and those with lower Gleason scores, first-line docetaxel for younger patients and those with more advanced disease, and first-line enzalutamide for patients with fewer metastases and more favourable performance status. The benefit to patients from these observations remains unknown.
Keywords: Abiraterone acetate; Androgen deprivation therapy; Antiandrogen; Cabazitaxel; Castration-resistant prostate cancer; Docetaxel; Enzalutamide; Ra-223; Real-world evidence.
© 2022 The Authors.
Figures

Similar articles
-
Matching-adjusted indirect comparison of talazoparib plus enzalutamide versus abiraterone acetate and docetaxel in mCRPC.Future Oncol. 2025 Apr;21(9):1075-1084. doi: 10.1080/14796694.2025.2471200. Epub 2025 Mar 5. Future Oncol. 2025. PMID: 40045559 Free PMC article.
-
Outcomes of First-Line Abiraterone Acetate or Enzalutamide for Older Adults With Metastatic Castration-Resistant Prostate Cancer According to Use of Upfront Docetaxel for Metastatic Castration-Sensitive Prostate Cancer in an International Multicenter Registry: A SPARTACUSS-Meet-URO 26 Study.Clin Genitourin Cancer. 2024 Oct;22(5):102185. doi: 10.1016/j.clgc.2024.102185. Epub 2024 Aug 9. Clin Genitourin Cancer. 2024. PMID: 39217072
-
Real-world Outcomes of Sequential Androgen-receptor Targeting Therapies with or Without Interposed Life-prolonging Drugs in Metastatic Castration-resistant Prostate Cancer: Results from the Dutch Castration-resistant Prostate Cancer Registry.Eur Urol Oncol. 2021 Aug;4(4):618-627. doi: 10.1016/j.euo.2019.09.005. Epub 2019 Oct 8. Eur Urol Oncol. 2021. PMID: 31601523
-
Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry.Target Oncol. 2020 Jun;15(3):301-315. doi: 10.1007/s11523-020-00720-2. Target Oncol. 2020. PMID: 32500294 Free PMC article.
-
Comparison of Abiraterone Acetate and Docetaxel with Androgen Deprivation Therapy in High-risk and Metastatic Hormone-naïve Prostate Cancer: A Systematic Review and Network Meta-analysis.Eur Urol. 2018 Jun;73(6):834-844. doi: 10.1016/j.eururo.2017.10.002. Epub 2017 Oct 14. Eur Urol. 2018. PMID: 29037513
Cited by
-
Evaluation of Second-Line Treatment for Castration-Resistant Prostate Cancer following the Administration of Upfront Androgen Receptor Signaling Inhibitors.Prostate Cancer. 2024 Aug 5;2024:9303603. doi: 10.1155/2024/9303603. eCollection 2024. Prostate Cancer. 2024. PMID: 39135744 Free PMC article.
-
Real-world treatment patterns and survival outcomes in men with metastatic castration-resistant prostate cancer in Finland: a national, population-based cohort study.Acta Oncol. 2025 Jan 29;64:173-178. doi: 10.2340/1651-226X.2025.42173. Acta Oncol. 2025. PMID: 39881601 Free PMC article.
-
A Bayesian Network Meta-analysis of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer in First- and Subsequent Lines.Target Oncol. 2025 Jul;20(4):569-595. doi: 10.1007/s11523-025-01148-2. Epub 2025 Jun 10. Target Oncol. 2025. PMID: 40493311 Free PMC article.
-
Azolato-Bridged Dinuclear Platinum(II) Complexes Exhibit Androgen Receptor-Mediated Anti-Prostate Cancer Activity.Inorg Chem. 2024 Nov 4;63(44):20951-20963. doi: 10.1021/acs.inorgchem.4c01093. Epub 2024 Sep 11. Inorg Chem. 2024. PMID: 39258898 Free PMC article.
-
Androgen-ablative therapies inducing CXCL8 regulates mTORC1/SREBP2-dependent cholesterol biosynthesis to support progression of androgen receptor negative prostate cancer cells.Oncogene. 2024 Nov;43(47):3456-3468. doi: 10.1038/s41388-024-03181-3. Epub 2024 Oct 5. Oncogene. 2024. PMID: 39369166
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- World Health Organization International Agency for Research on Cancer. Cancer today: prostate cancer. 2020. https://gco.iarc.fr/today/home.
-
- Lorente D., Mateo J., Perez-Lopez R., de Bono J.S., Attard G. Sequencing of agents in castration-resistant prostate cancer. Lancet Oncol. 2015;16:e279–e292. - PubMed
-
- Nuhn P., De Bono J.S., Fizazi K., et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99. - PubMed
LinkOut - more resources
Full Text Sources

