Drug-Drug-Gene Interactions in Cardiovascular Medicine
- PMID: 36353710
- PMCID: PMC9639705
- DOI: 10.2147/PGPM.S338601
Drug-Drug-Gene Interactions in Cardiovascular Medicine
Abstract
Cardiovascular disease remains a leading cause of both morbidity and mortality worldwide. It is widely accepted that both concomitant medications (drug-drug interactions, DDIs) and genomic factors (drug-gene interactions, DGIs) can influence cardiovascular drug-related efficacy and safety outcomes. Although thousands of DDI and DGI (aka pharmacogenomic) studies have been published to date, the literature on drug-drug-gene interactions (DDGIs, cumulative effects of DDIs and DGIs) remains scarce. Moreover, multimorbidity is common in cardiovascular disease patients and is often associated with polypharmacy, which increases the likelihood of clinically relevant drug-related interactions. These, in turn, can lead to reduced drug efficacy, medication-related harm (adverse drug reactions, longer hospitalizations, mortality) and increased healthcare costs. To examine the extent to which DDGIs and other interactions influence efficacy and safety outcomes in the field of cardiovascular medicine, we review current evidence in the field. We describe the different categories of DDIs and DGIs before illustrating how these two interact to produce DDGIs and other complex interactions. We provide examples of studies that have reported the prevalence of clinically relevant interactions and the most implicated cardiovascular medicines before outlining the challenges associated with dealing with these interactions in clinical practice. Finally, we provide recommendations on how to manage the challenges including but not limited to expanding the scope of drug information compendia, interaction databases and clinical implementation guidelines (to include clinically relevant DDGIs and other complex interactions) and work towards their harmonization; better use of electronic decision support tools; using big data and novel computational techniques; using clinically relevant endpoints, preemptive genotyping; ensuring ethnic diversity; and upskilling of clinicians in pharmacogenomics and personalized medicine.
Keywords: drug–drug interactions; drug–drug–gene interactions; drug–gene interactions; drug–gene–gene interactions; pharmacogenomics.
© 2022 Asiimwe and Pirmohamed.
Conflict of interest statement
MP has received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (co-funded by MRC and Roche, UCB, Eli Lilly and Novartis); and a PhD studentship jointly funded by EPSRC and AstraZeneca. He also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb. He has developed an HLA genotyping panel with MC Diagnostics, but does not benefit financially from this. He is part of the IMI Consortium ARDAT (www.ardat.org). None of the funding MP received is related to the current paper. IGA reports no conflicts of interest in this work.
Figures

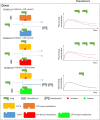
Similar articles
-
How common are drug and gene interactions? Prevalence in a sample of 1143 patients with CYP2C9, CYP2C19 and CYP2D6 genotyping.Pharmacogenomics. 2014 Apr;15(5):655-65. doi: 10.2217/pgs.14.6. Pharmacogenomics. 2014. PMID: 24798722
-
Multimorbidity, polypharmacy, and drug-drug-gene interactions following a non-ST elevation acute coronary syndrome: analysis of a multicentre observational study.BMC Med. 2020 Nov 25;18(1):367. doi: 10.1186/s12916-020-01827-z. BMC Med. 2020. PMID: 33234119 Free PMC article.
-
Prevalence and factors associated with potential clinically significant drug-drug interactions in patients with cardiovascular diseases at hospital admission.Acta Pharm. 2025 Jan 9;74(4):693-708. doi: 10.2478/acph-2024-0038. Print 2024 Dec 1. Acta Pharm. 2025. PMID: 39787625
-
Insights into pharmacogenetics, drug-gene interactions, and drug-drug-gene interactions.Drug Metab Rev. 2024 Aug 18:1-19. doi: 10.1080/03602532.2024.2385928. Online ahead of print. Drug Metab Rev. 2024. PMID: 39154360 Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Genetics in Ischemic Stroke: Current Perspectives and Future Directions.J Cardiovasc Dev Dis. 2023 Dec 13;10(12):495. doi: 10.3390/jcdd10120495. J Cardiovasc Dev Dis. 2023. PMID: 38132662 Free PMC article. Review.
-
Pharmacogenomics of Cardiovascular Drugs for Atherothrombotic, Thromboembolic and Atherosclerotic Risk.Genes (Basel). 2023 Nov 9;14(11):2057. doi: 10.3390/genes14112057. Genes (Basel). 2023. PMID: 38003001 Free PMC article. Review.
-
Genotyping of Patients with Adverse Drug Reaction or Therapy Failure: Database Analysis of a Pharmacogenetics Case Series Study.Pharmgenomics Pers Med. 2023 Jul 3;16:693-706. doi: 10.2147/PGPM.S415259. eCollection 2023. Pharmgenomics Pers Med. 2023. PMID: 37426898 Free PMC article.
-
Impact and Enablers of Pharmacogenetic-Informed Treatment Decisions-A Longitudinal Mixed-Methods Study Exploring the Patient Perspective.Pharmacy (Basel). 2025 Jan 31;13(1):14. doi: 10.3390/pharmacy13010014. Pharmacy (Basel). 2025. PMID: 39998012 Free PMC article.
-
Landscape of pharmacogenetic variants associated with non-insulin antidiabetic drugs in the Indian population.BMJ Open Diabetes Res Care. 2024 Mar 12;12(2):e003769. doi: 10.1136/bmjdrc-2023-003769. BMJ Open Diabetes Res Care. 2024. PMID: 38471670 Free PMC article.
References
-
- The Academy of Medical Sciences. Multimorbidity: a priority for global health research; 2018.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

