ATM Regulates Differentiation of Myofibroblastic Cancer-Associated Fibroblasts and Can Be Targeted to Overcome Immunotherapy Resistance
- PMID: 36353752
- PMCID: PMC9755965
- DOI: 10.1158/0008-5472.CAN-22-0435
ATM Regulates Differentiation of Myofibroblastic Cancer-Associated Fibroblasts and Can Be Targeted to Overcome Immunotherapy Resistance
Abstract
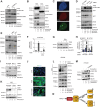
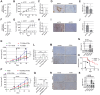
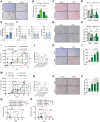
Myofibroblastic cancer-associated fibroblast (myoCAF)-rich tumors generally contain few T cells and respond poorly to immune-checkpoint blockade. Although myoCAFs are associated with poor outcome in most solid tumors, the molecular mechanisms regulating myoCAF accumulation remain unclear, limiting the potential for therapeutic intervention. Here, we identify ataxia-telangiectasia mutated (ATM) as a central regulator of the myoCAF phenotype. Differentiating myofibroblasts in vitro and myoCAFs cultured ex vivo display activated ATM signaling, and targeting ATM genetically or pharmacologically could suppress and reverse differentiation. ATM activation was regulated by the reactive oxygen species-producing enzyme NOX4, both through DNA damage and increased oxidative stress. Targeting fibroblast ATM in vivo suppressed myoCAF-rich tumor growth, promoted intratumoral CD8 T-cell infiltration, and potentiated the response to anti-PD-1 blockade and antitumor vaccination. This work identifies a novel pathway regulating myoCAF differentiation and provides a rationale for using ATM inhibitors to overcome CAF-mediated immunotherapy resistance.
Significance: ATM signaling supports the differentiation of myoCAFs to suppress T-cell infiltration and antitumor immunity, supporting the potential clinical use of ATM inhibitors in combination with checkpoint inhibition in myoCAF-rich, immune-cold tumors.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures
References
-
- Fearon DT. Immune-suppressing cellular elements of the tumor microenvironment. Ann Rev Cancer Biol 2017;1:241–55.
-
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. - PubMed
-
- Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol 2011;223:470–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

