Overexpression of Homer1b/c induces valproic acid resistance in epilepsy
- PMID: 36353757
- PMCID: PMC9804053
- DOI: 10.1111/cns.14008
Overexpression of Homer1b/c induces valproic acid resistance in epilepsy
Erratum in
-
Correction to "Overexpression of Homer1b/c induces valproic acid resistance in epilepsy".CNS Neurosci Ther. 2024 May;30(5):e14785. doi: 10.1111/cns.14785. CNS Neurosci Ther. 2024. PMID: 38797971 Free PMC article. No abstract available.
Abstract
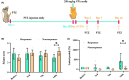
Aims: Resistance to valproic acid (VPA) is a major challenge for epilepsy treatment. We aimed to explore the mechanism underlying this resistance.
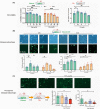
Methods: Pentylenetetrazol-induced chronic epileptic rats were administered VPA (250 mg/Kg) for 14 days; rats with controlled seizure stages (seizure score14th-before ≤0) and latent time (latent time14th-before ≥0) were considered VPA-responsive, while the others were considered nonresponsive. Differentially expressed genes (DEGs) between the VPA-responsive and nonresponsive rat hippocampus transcriptomes were identified, and their functions were evaluated. The roles of postsynaptic density (PSD) and Homer1 were also determined. Furthermore, a subtype of Homer1 (Homer1b/c) was overexpressed or silenced in HT22 cells to determine its effect on VPA efficacy. Moreover, the membrane levels of mGluR1/5 directly bound to Homer1b/c were assessed.
Results: Overall, 264 DEGs commonly enriched in the PSD between VPA-responsive and nonresponsive rats. Among them, Homer1 was more highly expressed in the hippocampus of nonresponses compared to that of responses. Overexpression of Homer1b/c interrupted VPA efficacy by increasing reactive oxygen species production, lactate dehydrogenase release, and calcium content. Furthermore, it induced the overexpression of mGluR1 and mGluR5.
Conclusion: Overexpression of Homer1b/c influenced VPA efficacy, revealing it could be a target to improve the efficacy of this treatment.
Keywords: HT22; Homer1b/c; VPA; efficacy; pentylenetetrazol.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
-
- Singh A, Trevick S. The Epidemiology of Global Epilepsy. Neurol Clin. 2016;34(4):837‐847. - PubMed
-
- Voll A, Hernandez‐Ronquillo L, Buckley S, Tellez‐Zenteno JF. Predicting drug resistance in adult patients with generalized epilepsy: A case‐control study. Epilepsy Behav. 2015;53:126‐130. - PubMed
-
- Janson MT, Bainbridge JL. Continuing Burden of Refractory Epilepsy. Ann Pharmacother. 2021;55(3):406‐408. - PubMed
-
- Rakitin A, Koks S, Haldre S. Valproate modulates glucose metabolism in patients with epilepsy after first exposure. Epilepsia. 2015;56(11):e172‐e175. - PubMed

