Sense-B-noise: an enigmatic cause for inappropriate shocks in subcutaneous implantable cardioverter defibrillators
- PMID: 36353759
- PMCID: PMC9935013
- DOI: 10.1093/europace/euac202
Sense-B-noise: an enigmatic cause for inappropriate shocks in subcutaneous implantable cardioverter defibrillators
Abstract
Aims: Subcutaneous implantable cardioverter defibrillators (S-ICDs) are well established. However, inappropriate shocks (IAS) remain a source of concern since S-ICDs offer very limited troubleshooting options. In our multicentre case series, we describe several patients who experienced IAS due to a previously unknown S-ICD system issue.
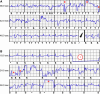
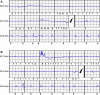
Methods and results: We observed six patients suffering from this novel IAS entity. The IAS occurred exclusively in primary or alternate S-ICD sensing vector configuration (therefore called 'Sense-B-noise'). IAS were caused by non-physiologic oversensing episodes characterized by intermittent signal saturation, diminished QRS amplitudes, and disappearance of the artefacts after the IAS. Noise/oversensing could not be provoked by manipulation, X-ray did not show evidence for lead/header issues and impedance measurements were within normal limits. The pooled experience of our centres implies that up to ∼5% of S-ICDs may be affected. The underlying root cause was discussed extensively with the manufacturer but remains unknown and is under further investigation.
Conclusion: Sense-B-noise is a novel cause for IAS due to non-physiologic signal oversensing, arising from a previously unknown S-ICD system issue. Sense-B-noise may be suspected if episodes of signal saturation in primary or alternate vector configuration are present, oversensing cannot be provoked, and X-ray and electrical measurements appear normal. The issue can be resolved by reprogramming the device to secondary sensing vector.
Keywords: Device failure; Inappropriate shock; Oversensing; S-ICD; Subcutaneous defibrillator.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None of the authors has received any compensation for this study. Haeberlin has received travel/educational grants from Medtronic and Philips/Spectranetics. He is consultant/advisor for DiNAQOR and Biotronik and Co-founder/head of Act-Inno. Burri has received institutional fellowship support and/or speaker fees from Abbott, Biotronik, Boston Scientific, Medtronic, and Microport. Schaer has received fees from Medtronic’s speaker’s bureau. Koepfli has received consultant fees from Microport, and speaker fees von Medtronic, Abbott Medical und Daiichi Sankyo. Grebmer received speaker fees from Abbott and Zoll, and received travel support from Biotronik. Breitenstein has received consultant and/or speaker fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Pfizer, and Spectranetics/Philipps. Reichlin has received consulting fees/speaker honoraria/travel support from Abbott, Astra Zeneca, Brahms, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Medtronic, Pfizer-BMS, and Roche. Noti has received travel/educational grants from Medtronic and Abbott, Boston Scientific and Philips/Spectranetics and speaker honoraria from Medtronic and Abbott.
Figures



References
-
- Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–53. - PubMed
-
- Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil Pet al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol 2017;70:830–41. - PubMed
-
- Knops RE, Olde Nordkamp LRA, Delnoy P-PHM, Boersma LVA, Kuschyk J, El-Chami MF et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–36. - PubMed

