Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson's disease
- PMID: 36353934
- PMCID: PMC10100513
- DOI: 10.1002/glia.24295
Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson's disease
Abstract
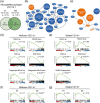
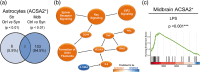
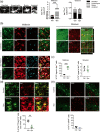
Inflammation is a common feature in neurodegenerative diseases that contributes to neuronal loss. Previously, we demonstrated that the basal inflammatory tone differed between brain regions and, consequently, the reaction generated to a pro-inflammatory stimulus was different. In this study, we assessed the innate immune reaction in the midbrain and in the striatum using an experimental model of Parkinson's disease. An adeno-associated virus serotype 9 expressing the α-synuclein and mCherry genes or the mCherry gene was administered into the substantia nigra. Myeloid cells (CD11b+ ) and astrocytes (ACSA2+ ) were purified from the midbrain and striatum for bulk RNA sequencing. In the parkinsonian midbrain, CD11b+ cells presented a unique anti-inflammatory transcriptomic profile that differed from degenerative microglia signatures described in experimental models for other neurodegenerative conditions. By contrast, striatal CD11b+ cells showed a pro-inflammatory state and were similar to disease-associated microglia. In the midbrain, a prominent increase of infiltrated monocytes/macrophages was observed and, together with microglia, participated actively in the phagocytosis of dopaminergic neuronal bodies. Although striatal microglia presented a phagocytic transcriptomic profile, morphology and cell density was preserved and no active phagocytosis was detected. Interestingly, astrocytes presented a pro-inflammatory fingerprint in the midbrain and a low number of differentially displayed transcripts in the striatum. During α-synuclein-dependent degeneration, microglia and astrocytes experience context-dependent activation states with a different contribution to the inflammatory reaction. Our results point towards the relevance of selecting appropriate cell targets to design neuroprotective strategies aimed to modulate the innate immune system during the active phase of dopaminergic degeneration.
Keywords: Parkinson's disease; astrocyte; inflammation; microglia; myeloid; neurodegeneration; synuclein.
© 2022 The Authors. GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Comment in
-
Glial response to Parkinson disease-like neurodegeneration is region-dependent.Nat Rev Neurol. 2023 Jan;19(1):2. doi: 10.1038/s41582-022-00754-2. Nat Rev Neurol. 2023. PMID: 36446915 No abstract available.
References
-
- Abellanas, M. A. , Zamarbide, M. , Basurco, L. , Luquin, E. , Garcia‐Granero, M. , Clavero, P. , San Martin‐Uriz, P. , Vilas, A. , Mengua, E. , Hervas‐Stubbs, S. , & Aymerich, M. S. (2019). Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. Journal of Neuroinflammation, 16, 233. - PMC - PubMed
-
- A‐Gonzalez, N. , Bensinger, S. J. , Hong, C. , Beceiro, S. , Bradley, M. N. , Zelcer, N. , Deniz, J. , Ramirez, C. , Díaz, M. , Gallardo, G. , de Galarreta, C. R. , Salazar, J. , Lopez, F. , Edwards, P. , Parks, J. , Andujar, M. , Tontonoz, P. , & Castrillo, A. (2009). Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity, 31, 245–258. - PMC - PubMed
-
- Ahmed, I. , Tamouza, R. , Delord, M. , Krishnamoorthy, R. , Tzourio, C. , Mulot, C. , Nacfer, M. , Lambert, J. C. , Beaune, P. , Laurent‐Puig, P. , Loriot, M. A. , Charron, D. , & Elbaz, A. (2012). Association between Parkinson's disease and the HLA‐DRB1 locus. Movement Disorders, 27, 1104–1110. - PubMed
-
- Batiuk, M. Y. , Martirosyan, A. , Wahis, J. , de Vin, F. , Marneffe, C. , Kusserow, C. , Koeppen, J. , Viana, J. F. , Oliveira, J. F. , Voet, T. , Ponting, C. P. , Belgard, T. G. , & Holt, M. G. (2020). Identification of region‐specific astrocyte subtypes at single cell resolution. Nature Communications, 11, 1220. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- BFU2017-90043-P/Agencia Estatal de Investigación
- PC 060-061/Dirección General de Industria y Energia, Gobierno de Navarra. Proyectos Colaborativos
- PC 192-193/Dirección General de Industria y Energia, Gobierno de Navarra. Proyectos Colaborativos
- Fundacion Gangoiti
- PI20/01063/Instituto de Salud Carlos III-FEDER
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

