Internal RNA 2'O-methylation in the HIV-1 genome counteracts ISG20 nuclease-mediated antiviral effect
- PMID: 36354007
- PMCID: PMC10085690
- DOI: 10.1093/nar/gkac996
Internal RNA 2'O-methylation in the HIV-1 genome counteracts ISG20 nuclease-mediated antiviral effect
Abstract
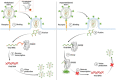
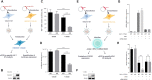
RNA 2'O-methylation is a 'self' epitranscriptomic modification allowing discrimination between host and pathogen. Indeed, human immunodeficiency virus 1 (HIV-1) induces 2'O-methylation of its genome by recruiting the cellular FTSJ3 methyltransferase, thereby impairing detection by RIG-like receptors. Here, we show that RNA 2'O-methylations interfere with the antiviral activity of interferon-stimulated gene 20-kDa protein (ISG20). Biochemical experiments showed that ISG20-mediated degradation of 2'O-methylated RNA pauses two nucleotides upstream of and at the methylated residue. Structure-function analysis indicated that this inhibition is due to steric clash between ISG20 R53 and D90 residues and the 2'O-methylated nucleotide. We confirmed that hypomethylated HIV-1 genomes produced in FTSJ3-KO cells were more prone to in vitro degradation by ISG20 than those produced in cells expressing FTSJ3. Finally, we found that reverse-transcription of hypomethylated HIV-1 was impaired in T cells by interferon-induced ISG20, demonstrating the direct antagonist effect of 2'O-methylation on ISG20-mediated antiviral activity.
Plain language summary
Despite highly effective antiretroviral therapies, the human immunodeficiency virus (HIV-1) remains a major public health threat. Its pathogenesis depends on its ability to establish a persistent infection in cells of the immune system. Our study highlights a new insight into how HIV-1 evades early restriction by the immune system. We showed that 2′O-methylation marks found inside HIV-1 RNA promote viral evasion from the antiviral action of the interferon-stimulated gene 20-kDa protein (ISG20), an innate immune restriction factor with a nuclease activity. By disrupting the level of 2′O-methylation of the HIV-1 genome, we demonstrated that ISG20 impairs the reverse transcription process of hypomethylated viruses, as a result of viral RNA decay.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





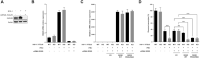
References
-
- McIntyre W., Netzband R., Bonenfant G., Biegel J.M., Miller C., Fuchs G., Henderson E., Arra M., Canki M., Fabris D.et al. .. Positive-sense RNA viruses reveal the complexity and dynamics of the cellular and viral epitranscriptomes during infection. Nucleic Acids Res. 2018; 46:5776–5791. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

