CotG Mediates Spore Surface Permeability in Bacillus subtilis
- PMID: 36354330
- PMCID: PMC9765600
- DOI: 10.1128/mbio.02760-22
CotG Mediates Spore Surface Permeability in Bacillus subtilis
Abstract
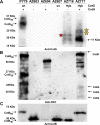
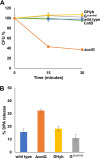
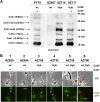
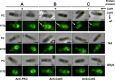
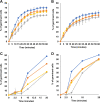
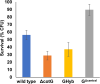
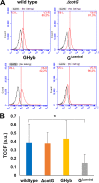
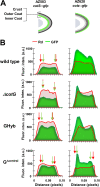
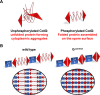
Proteins and glycoproteins that form the surface layers of the Bacillus spore assemble into semipermeable arrays that surround and protect the spore cytoplasm. Such layers, acting like molecular sieves, exclude large molecules but allow small nutrients (germinants) to penetrate. We report that CotG, a modular and abundant component of the Bacillus subtilis spore coat, controls spore permeability through its central region, formed by positively charged tandem repeats. These repeats act as spacers between the N and C termini of the protein, which are responsible for the interaction of CotG with at least one other coat protein. The deletion but not the replacement of the central repeats with differently charged repeats affects the spore resistance to lysozyme and the efficiency of germination-probably by reducing the coat permeability to external molecules. The presence of central repeats is a common feature of the CotG-like proteins present in most Bacillus species, and such a wide distribution of this protein family is suggestive of a relevant role for the structure and function of the Bacillus spore. IMPORTANCE Bacterial spores are quiescent cells extremely resistant to a variety of unphysiological conditions, including the presence of lytic enzymes. Such resistance is also due to the limited permeability of the spore surface, which does not allow lytic enzymes to reach the spore interior. This article proposes that the spore permeability in B. subtilis is mediated by CotG, a modular protein formed by a central region of repeats of positively charged amino acid acting as a "spacer" between the N and C termini. These, in turn, interact with other coat proteins, generating a protein layer whose permeability to external molecules is controlled by the distance between the N and C termini of CotG. This working model is most likely expandable to most sporeformers of the Bacillus genus, since they all have CotG-like proteins, not homologous to CotG of B. subtilis but similarly characterized by central repeats.
Keywords: Bacillus subtilis; endospores; germination; permeability; spore coat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

