Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms
- PMID: 36354433
- PMCID: PMC9688173
- DOI: 10.3390/bios12110924
Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms
Abstract
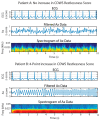
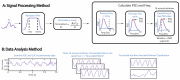
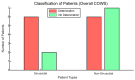
Treating opioid use disorder (OUD) is a significant healthcare challenge in the United States. Remaining abstinent from opioids is challenging for individuals with OUD due to withdrawal symptoms that include restlessness. However, to our knowledge, studies of acute withdrawal have not quantified restlessness using involuntary movements. We hypothesized that wearable accelerometry placed mid-sternum could be used to detect withdrawal-related restlessness in patients with OUD. To study this, 23 patients with OUD undergoing active withdrawal participated in a protocol involving wearable accelerometry, opioid cues to elicit craving, and non-invasive Vagal Nerve Stimulation (nVNS) to dampen withdrawal symptoms. Using accelerometry signals, we analyzed how movements correlated with changes in acute withdrawal severity, measured by the Clinical Opioid Withdrawal Scale (COWS). Our results revealed that patients demonstrating sinusoidal-i.e., predominantly single-frequency oscillation patterns in their motion almost exclusively demonstrated an increase in the COWS, and a strong relationship between the maximum power spectral density and increased withdrawal over time, measured by the COWS (R = 0.92, p = 0.029). Accelerometry may be used in an ambulatory setting to indicate the increased intensity of a patient's withdrawal symptoms, providing an objective, readily-measurable marker that may be captured ubiquitously.
Keywords: accelerometer; non-invasive therapies; opioid addiction; opioid use disorder; opioid withdrawal; restlessness; spectral analysis; spectrograms; vagal nerve stimulation; wearable.
Conflict of interest statement
The active and sham vagus nerve stimulation devices used in this research were provided free of charge by electroCore, Inc. J.D.B. has received research funding support and device support from electroCore LLC, is co-investigator with investigators from Evren Technologies on a Department of Defense grant and serves on the Scientific Advisory Board for Evren Technologies, Inc. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institute on Drug Abuse or the National Science Foundation.
Figures

References
-
- NIDA Overdose Death Rates. [(accessed on 22 November 2021)]; Available online: https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-r....
MeSH terms
Substances
Grants and funding
- I01 RX003418/RX/RRD VA/United States
- I01 CX002331/CX/CSRD VA/United States
- R01 MH056120/MH/NIMH NIH HHS/United States
- UG3 DA048502-01A1S1/DA/NIDA NIH HHS/United States
- R01 MH120262/MH/NIMH NIH HHS/United States
- DGE-2039655/National Science Foundation
- UH3 DA048502/DA/NIDA NIH HHS/United States
- UG3 DA048502/DA/NIDA NIH HHS/United States
- K24 MH076955/MH/NIMH NIH HHS/United States
- R01 HL088726/HL/NHLBI NIH HHS/United States
- UG3 DA048502-01A1S2/DA/NIDA NIH HHS/United States
- P01 HL101398/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

