Electrochemical Determination of Morphine in Urine Samples by Tailoring FeWO4/CPE Sensor
- PMID: 36354441
- PMCID: PMC9688003
- DOI: 10.3390/bios12110932
Electrochemical Determination of Morphine in Urine Samples by Tailoring FeWO4/CPE Sensor
Abstract
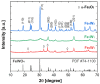
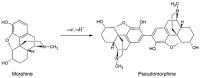
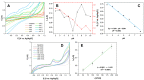
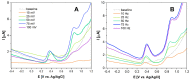
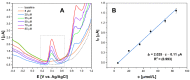
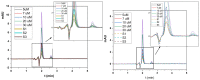
Morphine (MORPH) is natural alkaloid and mainly used as a pain reliever. Its monitoring in human body fluids is crucial for modern medicine. In this paper, we have developed an electrochemical sensor for submicromolar detection of MORPH. The sensor is based on modified carbon paste electrode (CPE) by investigating the FexW1-xO4 ratio in iron tungstate (FeWO4), as well as the ratio of this material in CPE. For the first time, the effect of the iron-tungsten ratio in terms of achieving the best possible electrochemical characteristics for the detection of an important molecule for humans was examined. Morphological and electrochemical characteristics of materials were studied. The best results were obtained using Fe1W3 and 7.5% of modifier in CPE. For MORPH detection, square wave voltammetry (SWV) was optimized. Under the optimized conditions, Fe1W3@CPE resulted in limit of detection (LOD) of the method of 0.58 µM and limit of quantification (LOQ) of 1.94 µM. The linear operating range between 5 and 85 µM of MORPH in the Britton-Robinson buffer solution (BRBS) at pH 8 as supporting electrolyte was obtained. The Fe1W3@CPE sensor resulted in good selectivity and excellent repeatability with relative standard deviation (RSD) and was applied in real-world samples of human urine. Application for direct MORPH detection, without tedious sample pretreatment procedures, suggests that developed electrochemical sensor has appeared to be a suitable competitor for efficient, precise, and accurate monitoring of the MORPH in biological fluids.
Keywords: carbon paste electrode; electroanalysis; iron tungstate; morphine; real-world sample; square wave voltammetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rajaei M., Foroughi M.M., Jahani S., Shahidi Zandi M., Hassani Nadiki H. Sensitive detection of morphine in the presence of dopamine with La3+ doped fern-like CuO nanoleaves/MWCNTs modified carbon paste electrode. J. Mol. Liq. 2019;284:462–472. doi: 10.1016/j.molliq.2019.03.135. - DOI
-
- Wester N., Mynttinen E., Etula J., Lilius T., Kalso E., Kauppinen E.I., Laurila T., Koskinen J. Simultaneous Detection of Morphine and Codeine in the Presence of Ascorbic Acid and Uric Acid and in Human Plasma at Nafion Single-Walled Carbon Nanotube Thin-Film Electrode. ACS Omega. 2019;4:17726–17734. doi: 10.1021/acsomega.9b02147. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

