Lateral Flow Microimmunoassay (LFµIA) for the Reliable Quantification of Allergen Traces in Food Consumables
- PMID: 36354489
- PMCID: PMC9688043
- DOI: 10.3390/bios12110980
Lateral Flow Microimmunoassay (LFµIA) for the Reliable Quantification of Allergen Traces in Food Consumables
Abstract
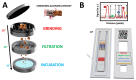
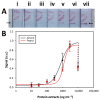
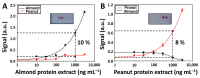
Quality assurance and food safety are of great concern within the food industry because of unknown quantities of allergens often present in food. Therefore, there is an ongoing need to develop rapid, sensitive, and easy to use methods that serve as an alternative to mass spectrometry and enzyme-linked immunosorbent assay (ELISA) for monitoring food safety. Lateral flow immunoassay is one of the most used point-of-need devices for clinical, environmental, and food safety applications. Compared to traditional methods, it appears to be a simple and fast alternative for detecting food allergens. However, its reliability is frequently questioned due to the lack of quantitative information. In this study, a lateral flow microimmunoassay (LFµIA) is presented that integrates up to 36 spots in microarray format in a single strip, providing semi-quantitative information about the level of allergens, positive and negative controls, internal calibration, and hook effect. The LFµIA has been evaluated for the on-site simultaneous and reliable quantification of almond and peanut allergens as a proof of concept, demonstrating high sensitivity (185 and 229 µg/kg, respectively), selectivity (77%), and accuracy (RSD 5-25%) when analyzing commercial allergen-suspicious food consumables.
Keywords: food-borne allergens; internal calibration; lateral flow immunoassay; microarray; multiplexing; nanoparticles; smartphone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ebisawa M., Pawankar R. Food Allergy—A Rising Global Health Problem; Proceedings of the World Allergy Week; Virtual. 8–14 April 2013.
-
- Protect Yourself from Food Allergies. [(accessed on 18 June 2021)]; Available online: https://www.foodsafety.gov/blog/protect-yourself-food-allergies.
-
- United States Anaphylaxis Markets, 2020–2021 & Forecast to 2027. [(accessed on 18 June 2021)]. Available online: https://www.prnewswire.com/news-releases/united-states-anaphylaxis-marke....
-
- r-biopharm RIDA®QUICK Gliadin Art. No. R7003. [(accessed on 18 June 2021)]. Available online: https://food.r-biopharm.com/es/productos/ridaquick-gliadin2/
-
- Romerlabs AgraStrip: Allergen Lateral Flow Devices. [(accessed on 18 June 2021)]. Available online: https://www.romerlabs.com/shop/inter_en/test-kits/lateral-flow-devices/a...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

