A Novel 3D Printing Particulate Manufacturing Technology for Encapsulation of Protein Therapeutics: Sprayed Multi Adsorbed-Droplet Reposing Technology (SMART)
- PMID: 36354564
- PMCID: PMC9687125
- DOI: 10.3390/bioengineering9110653
A Novel 3D Printing Particulate Manufacturing Technology for Encapsulation of Protein Therapeutics: Sprayed Multi Adsorbed-Droplet Reposing Technology (SMART)
Abstract
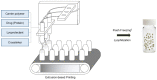
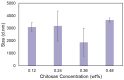
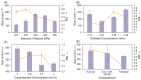
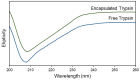
Recently, various innovative technologies have been developed for the enhanced delivery of biologics as attractive formulation targets including polymeric micro and nanoparticles. Combined with personalized medicine, this area can offer a great opportunity for the improvement of therapeutics efficiency and the treatment outcome. Herein, a novel manufacturing method has been introduced to produce protein-loaded chitosan particles with controlled size. This method is based on an additive manufacturing technology that allows for the designing and production of personalized particulate based therapeutic formulations with a precise control over the shape, size, and potentially the geometry. Sprayed multi adsorbed-droplet reposing technology (SMART) consists of the high-pressure extrusion of an ink with a well determined composition using a pneumatic 3D bioprinting approach and flash freezing the extrudate at the printing bed, optionally followed by freeze drying. In the present study, we attempted to manufacture trypsin-loaded chitosan particles using SMART. The ink and products were thoroughly characterized by dynamic light scattering, rheometer, Scanning Electron Microscopy (SEM), and Fourier Transform Infra-Red (FTIR) and Circular Dichroism (CD) spectroscopy. These characterizations confirmed the shape morphology as well as the protein integrity over the process. Further, the effect of various factors on the production were investigated. Our results showed that the concentration of the carrier, chitosan, and the lyoprotectant concentration as well as the extrusion pressure have a significant effect on the particle size. According to CD spectra, SMART ensured Trypsin's secondary structure remained intact regardless of the ink composition and pressure. However, our study revealed that the presence of 5% (w/v) lyoprotectant is essential to maintain the trypsin's proteolytic activity. This study demonstrates, for the first time, the viability of SMART as a single-step efficient process to produce biologics-based stable formulations with a precise control over the particulate morphology which can further be expanded across numerous therapeutic modalities including vaccines and cell/gene therapies.
Keywords: SMART; chitosan nanoparticle; ionic gelation; particle manufacturing; protein encapsulation.
Conflict of interest statement
The authors declare the following conflicts of interest. All authors are co-inventors of related intellectual property (IP). M.M., an author of this manuscript, holds stock in, serves on a scientific advisory board for, or is a consultant for CoM3D Ltd., (Surrey, UK) and Septum Solutions LLC. (Jacksonville, TX, USA). The terms of this arrangement have been reviewed and approved by the University of Texas at Austin in accordance with its policy on objectivity in research. The authors, and specifically M.M., N.H.A., and A.L., would also like to acknowledge the financial support from CoM3D Ltd., under an existing Master Sponsored Research Agreement with the University of Texas at Austin.
Figures




Similar articles
-
A Proof-of-Concept Preparation of Lipid-Plasmid DNA Particles Using Novel Extrusion-Based 3D-Printing Technology, SMART.Mol Pharm. 2023 Dec 4;20(12):6504-6508. doi: 10.1021/acs.molpharmaceut.3c00783. Epub 2023 Nov 6. Mol Pharm. 2023. PMID: 37931027
-
Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method: An effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery.Mater Sci Eng C Mater Biol Appl. 2017 Jan 1;70(Pt 1):378-385. doi: 10.1016/j.msec.2016.09.003. Epub 2016 Sep 6. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 27770906
-
Chitosan based micro- and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods.Eur J Pharm Biopharm. 2015 May;92:112-9. doi: 10.1016/j.ejpb.2015.03.004. Epub 2015 Mar 11. Eur J Pharm Biopharm. 2015. PMID: 25769679
-
Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges.J Control Release. 2021 Apr 10;332:367-389. doi: 10.1016/j.jconrel.2021.02.027. Epub 2021 Feb 27. J Control Release. 2021. PMID: 33652114 Review.
-
Preparation of Smart Materials by Additive Manufacturing Technologies: A Review.Materials (Basel). 2021 Oct 27;14(21):6442. doi: 10.3390/ma14216442. Materials (Basel). 2021. PMID: 34771968 Free PMC article. Review.
Cited by
-
Fabrication of mRNA encapsulated lipid nanoparticles using state of the art SMART-MaGIC technology and transfection in vitro.Sci Rep. 2024 Sep 30;14(1):22714. doi: 10.1038/s41598-024-73804-y. Sci Rep. 2024. PMID: 39349578 Free PMC article.
-
Rising role of 3D-printing in delivery of therapeutics for infectious disease.J Control Release. 2024 Feb;366:349-365. doi: 10.1016/j.jconrel.2023.12.051. Epub 2024 Jan 8. J Control Release. 2024. PMID: 38182058 Free PMC article. Review.
References
-
- Ran R., Sun Q., Baby T., Wibowo D., Middelberg A.P., Zhao C.-X. Multiphase microfluidic synthesis of micro-and nanostructures for pharmaceutical applications. Chem. Eng. Sci. 2017;169:78–96. doi: 10.1016/j.ces.2017.01.008. - DOI
-
- Tong X., Pan W., Su T., Zhang M., Dong W., Qi X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020;148:104501. doi: 10.1016/j.reactfunctpolym.2020.104501. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

