Portable Quartz Crystal Resonator Sensor for Characterising the Gelation Kinetics and Viscoelastic Properties of Hydrogels
- PMID: 36354626
- PMCID: PMC9690109
- DOI: 10.3390/gels8110718
Portable Quartz Crystal Resonator Sensor for Characterising the Gelation Kinetics and Viscoelastic Properties of Hydrogels
Abstract
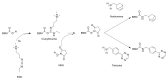
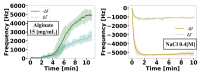
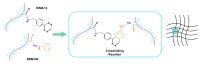
Hydrogel biomaterials have found use in various biomedical applications partly due to their biocompatibility and tuneable viscoelastic properties. The ideal rheological properties of hydrogels depend highly on the application and should be considered early in the design process. Rheometry is the most common method to study the viscoelastic properties of hydrogels. However, rheometers occupy much space and are costly instruments. On the other hand, quartz crystal resonators (QCRs) are devices that can be used as low-cost, small, and accurate sensors to measure the viscoelastic properties of fluids. For this reason, we explore the capabilities of a low-cost and compact QCR sensor to sense and characterise the gelation process of hydrogels while using a low sample amount and by sensing two different crosslink reactions: covalent bonds and divalent ions. The gelation of covalently crosslinked mucin hydrogels and physically crosslinked alginate hydrogels could be monitored using the sensor, clearly distinguishing the effect of several parameters affecting the viscoelastic properties of hydrogels, including crosslinking chemistry, polymer concentrations, and crosslinker concentrations. QCR sensors offer an economical and portable alternative method to characterise changes in a hydrogel material's viscous properties to contribute to this type of material design, thus providing a novel approach.
Keywords: covalently crosslinked hydrogels; hydrogel kinetics characterisation; physically crosslinked hydrogels; quartz crystal resonator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
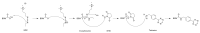
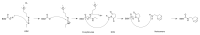
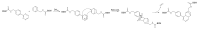


Similar articles
-
Crosslinker Architectures Impact Viscoelasticity in Dynamic Covalent Hydrogels.bioRxiv [Preprint]. 2024 Jun 4:2024.05.07.593040. doi: 10.1101/2024.05.07.593040. bioRxiv. 2024. Update in: Adv Healthc Mater. 2024 Dec;13(30):e2402059. doi: 10.1002/adhm.202402059. PMID: 38766044 Free PMC article. Updated. Preprint.
-
Effects of Hydroxyapatite Additions on Alginate Gelation Kinetics During Cross-Linking.Polymers (Basel). 2025 Jan 19;17(2):242. doi: 10.3390/polym17020242. Polymers (Basel). 2025. PMID: 39861314 Free PMC article.
-
Dynamic Mechanical Control of Alginate-Fibronectin Hydrogels with Dual Crosslinking: Covalent and Ionic.Polymers (Basel). 2021 Jan 29;13(3):433. doi: 10.3390/polym13030433. Polymers (Basel). 2021. PMID: 33573020 Free PMC article.
-
Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels.Acta Biomater. 2021 Apr 15;125:57-71. doi: 10.1016/j.actbio.2021.02.018. Epub 2021 Feb 16. Acta Biomater. 2021. PMID: 33601067 Review.
-
Dynamic covalent crosslinked hyaluronic acid hydrogels and nanomaterials for biomedical applications.Biomater Sci. 2022 Nov 8;10(22):6399-6412. doi: 10.1039/d2bm01154a. Biomater Sci. 2022. PMID: 36214100 Review.
Cited by
-
ANN-Based Discernment of Septic and Inflammatory Synovial Fluid: A Novel Method Using Viscosity Data from a QCR Sensor.Sensors (Basel). 2022 Dec 2;22(23):9413. doi: 10.3390/s22239413. Sensors (Basel). 2022. PMID: 36502129 Free PMC article.
-
Characterizing a Cost-Effective Hydrogel-Based Transparent Soil.Gels. 2023 Oct 21;9(10):835. doi: 10.3390/gels9100835. Gels. 2023. PMID: 37888408 Free PMC article.
References
-
- Deligkaris K., Tadele T.S., Olthuis W., van den Berg A. Hydrogel-based devices for biomedical applications. Sens. Actuators B Chem. 2010;147:765–774. doi: 10.1016/j.snb.2010.03.083. - DOI
-
- Yan H., Melin M., Jiang K., Trossbach M., Badadamath B., Langer K., Winkeljann B., Lieleg O., Hong J., Joensson H.N., et al. Immune-Modulating Mucin Hydrogel Microdroplets for the Encapsulation of Cell and Microtissue. Adv. Funct. Mater. 2021;31:2105967. doi: 10.1002/adfm.202105967. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

