The Study of the Influence of ZrO2 Precursor Type and the Temperature of Annealing on the Crystallization of the Tetragonal Polymorph of ZrO2 in Zirconia-Silica Gels
- PMID: 36354632
- PMCID: PMC9690174
- DOI: 10.3390/gels8110724
The Study of the Influence of ZrO2 Precursor Type and the Temperature of Annealing on the Crystallization of the Tetragonal Polymorph of ZrO2 in Zirconia-Silica Gels
Abstract
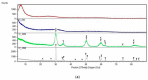
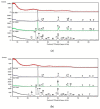
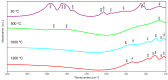
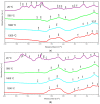
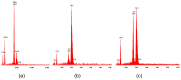
Materials of the ZrO2-SiO2 system were obtained by the sol-gel method applying two different types of ZrO2 precursors: zirconium (IV) n-propoxide Zr(OC3H7)4 and zirconium (IV) acetate Zr(OOC2H3)4 (organic acetic acid salt) while commonly used tetraethoxysilane TEOS was selected as SiO2 introducing one. ZrO2 concentration in synthesized samples varied from 20% to 50% (mol.). After drying for 28 days, all gels were annealed at 500 °C, 1000 °C, and 1200 °C in air. FTIR spectroscopy together with XRD diffraction was selected as the two main structure research methods. SEM microscopy was applied to analyze the local chemical compositions of samples and to observe the morphology of gels' surfaces. The analysis of FTIR spectra and XRD diffraction patterns allowed us to recognize different ZrO2 polymorphs which appeared in the samples depending strongly as well on ZrO2 precursor type as on the temperature of annealing. Samples synthesized by using the zirconium (IV) n-propoxide contained both cubic and tetragonal zirconia phases in general but showed the tendency of the increasing t-ZrO2 content in gels richer in ZrO2 and heated up to 1200 °C. However, in materials obtained applying zirconium (IV) acetate, the first detected at 500 °C phase was t-ZrO2 which was then conversing to m-ZrO2 form with the increasing temperature in case of samples rich in ZrO2. Meanwhile, t-ZrO2 was the predominant phase in samples of the lower content of ZrO2 but annealed at higher temperatures. By the analysis of changes in band profiles and positions, one can draw conclusions that the structure of studied samples is mostly built up of an amorphous silica matrix, in which different types of zirconia polymorphs create their own crystal lattice. The presence of the particular polymorph depends strongly on the type of zirconia precursor and the temperature of annealing.
Keywords: FTIR spectroscopy; SEM; X-ray diffraction; ZrO2-SiO2 system; sol-gel method; t-ZrO2.
Conflict of interest statement
The author declares no conflict of interest.
Figures








References
-
- Duhan S., Tomer V.K., Sharma A.K., Dehiya B.S. Development and properties study of microstructure silver-doped silica nanocomposites by chemical process. J. Alloys Compd. 2014;583:550–553. doi: 10.1016/j.jallcom.2013.09.018. - DOI
-
- Günter S., Budak S., Gibson B. Optical properties of Ag nanoclusters formed by irradiation and annealing of SiO2/SiO2/Ag thin films. Appl. Surf. Sci. 2014;310:180–183. doi: 10.1016/j.apsusc.2014.01.202. - DOI
-
- Zhang Q. ZrO2 thin films and ZrO2-SiO2 optical reflection filters deposited by sol–gel method. Mat. Lett. 2000;45:311–314. doi: 10.1016/S0167-577X(00)00124-5. - DOI
-
- Štefanć I.I., Musić S., Štefanić G., Gajović A. Thermal behavior of ZrO2 precursors obtained by sol-gel processing. J. Mol. Struct. 1999;480–481:621–625. doi: 10.1016/S0022-2860(98)00827-8. - DOI
-
- Kurpaska L., Favergeon J., Lahoche L., El-Marssi M., Grosseau Poussard J.-L., Moulin G., Roelandt J.-M. Raman Spectroscopy analysis of air grown oxide scale developed on pure zirconium substrate. J. Nucl. Mater. 2015;466:460–467. doi: 10.1016/j.jnucmat.2015.06.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

