A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy
- PMID: 36354641
- PMCID: PMC9689985
- DOI: 10.3390/gels8110733
A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy
Abstract
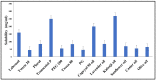
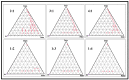
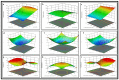
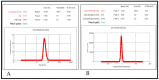
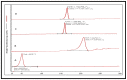
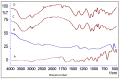
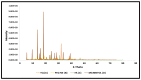
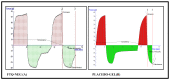
Thymoquinone has a multitude of pharmacological effects and has been researched for a wide variety of indications, but with limited clinical success. It is associated with pharmaco-technical caveats such as hydrophobicity, high degradation, and a low oral bioavailability. A prudent approach warrants its usage through an alternative dermal route in combination with functional excipients to harness its potential for treating dermal afflictions, such as psoriasis. Henceforth, the present study explores a nanoformulation approach for designing a fulvic acid (peat-sourced)-based thymoquinone nanoemulsion gel (FTQ-NEG) for an enhanced solubility and improved absorption. The excipients, surfactant/co-surfactant, and oil selected for the o/w nanoemulsion (FTQ-NE) are Tween 80/Transcutol-P and kalonji oil. The formulation methodology includes high-energy ultrasonication complemented with a three-dimensional/factorial Box-Behnken design for guided optimization. The surface morphology assessment through scanning/transmission electron microscopy and fluorescence microscopy revealed a 100 nm spherical, globule-like structure of the prepared nanoemulsion. Furthermore, the optimized FTQ-NE had a zeta potential of -2.83 ± 0.14 Mv, refractive index of 1.415 ± 0.036, viscosity of 138.5 ± 3.08 mp, and pH of 5.8 ± 0.16, respectively. The optimized FTQ-NE was then formulated as a gel using Carbopol 971® (1%). The in vitro release analysis of the optimized FTQ-NEG showed a diffusion-dominant drug release (Higuchi model) for 48 h. The drug permeation flux observed for FTQ-NEG (3.64 μg/cm2/h) was much higher compared to that of the pure drug (1.77 mg/cm2/h). The results were further confirmed by confocal microscopy studies, which proved the improved penetration of thymoquinone through mice skin. Long-term stability studies of the purported formulation were also conducted and yielded satisfactory results.
Keywords: Box–Behnken design; enhanced stability; nanoemulsion; pseudo-ternary phase diagrams; thymoquinone; ultrasonication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

