Monitoring the Methyl Eugenol Response and Non-Responsiveness Mechanisms in Oriental Fruit Fly Bactrocera dorsalis in China
- PMID: 36354828
- PMCID: PMC9695349
- DOI: 10.3390/insects13111004
Monitoring the Methyl Eugenol Response and Non-Responsiveness Mechanisms in Oriental Fruit Fly Bactrocera dorsalis in China
Abstract
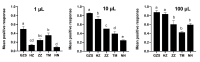
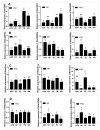
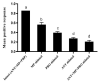
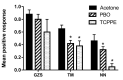
Bactrocera dorsalis is a notorious polyphagous pest in China, and its management strategies largely depend on methyl eugenol (ME), which has been widely used as an attractant to monitor and eradicate B. dorsalis populations for seven decades. However, the non-responsiveness levels in field B. dorsalis populations to ME is unknown. In this study, we monitored the response to ME in field populations from the four most heavily infested provinces in China, and the results showed that the populations had lower sensitivity to ME relative to GZS susceptible strain. The percent responsiveness of the lowest sensitivity population was 5.88-, 3.47-, and 1.47-fold lower relative to the susceptible strain at doses of 1, 10, and 100 µL of ME, respectively. Gene expression analysis and inhibitor assays further revealed that odorant binding protein (BdorOBP2, BdorOBP83b) and the P450 enzyme system may be associated with the lower response to ME. To our knowledge, this work is the first to report that the P450 enzyme system confers a lower responsiveness to lure insects. These findings provided valuable insights for exploiting ME non-responsiveness to protect sterile males from ME-based control strategies and the use of lures combined with insecticides.
Keywords: P450; methyl eugenol; non-responsiveness; odorant binding protein; trapping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Clarke A.R., Armstrong K.F., Carmichael A.E., Milne J.R., Raghu S., Roderick G.K., Yeates D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005;50:293–319. doi: 10.1146/annurev.ento.50.071803.130428. - DOI - PubMed
-
- Wei D., Liu Y.W., Zhang S.Y., Xu H.Q., Smagghe G., Wang J.J. A male accessory gland specific gene takeout2 regulates male mating success in Bactrocera dorsalis. Entomol. Gen. 2021;41:579–589. doi: 10.1127/entomologia/2021/1094. - DOI
-
- Zeng Y., Reddy G.V.P., Li Z., Qin Y., Wang Y., Pan X., Jiang F., Gao F., Zhao Z.-H. Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) J. Appl. Entomol. 2019;143:165–176. doi: 10.1111/jen.12582. - DOI
-
- Liu H., Zhang D.j., Xu Y., Wang L., Cheng D., Qi Y., Zeng L., Lu Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Integr. Agric. 2019;18:771–787. doi: 10.1016/S2095-3119(18)62015-5. - DOI
-
- Zhu Y.-F., Tan X.-M., Qi F.-J., Teng Z.-W., Fan Y.-J., Shang M.-Q., Lu Z.-Z., Wan F.-H., Zhou H.-X. The host shift of Bactrocera dorsalis: Early warning of the risk of damage to the fruit industry in northern China. Entomol. Gen. 2022;42:691–699. doi: 10.1127/entomologia/2022/1453. - DOI
Grants and funding
- 32102250/National Natural Science Foundation of China
- ZR2021QC017/Natural Science Foundation of Shandong Province, China
- 663/1121018/Scientific Research Fund for High-level Talents in Qingdao Agricultural University
- 2019JZZY010711/Major Scientific and Technological Innovation Projects in Shandong Province
LinkOut - more resources
Full Text Sources

