Efficacy of Bovine Nail Membranes as In Vitro Model for Onychomycosis Infected by Trichophyton Species
- PMID: 36354900
- PMCID: PMC9695884
- DOI: 10.3390/jof8111133
Efficacy of Bovine Nail Membranes as In Vitro Model for Onychomycosis Infected by Trichophyton Species
Abstract
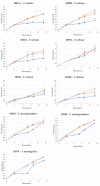
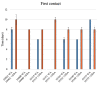
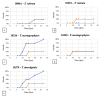
Onychomycosis is a fungal infection caused by different etiologic agents, including dermatophytes that specifically colonize keratin-rich substrates. The aim of this work was to investigate mechanical modifications of bovine membranes (used as an in vitro nail model) placed in contact with Trichophyton species. Trichophyton strains were isolated from toenails specimens. The procedure was set up by spreading T. rubrum,T. interdigitale, and T. mentagrophytes strains on Petri dishes with minimal and rich media; after that, bovine membranes were placed in the center. After 27 days, T. interdigitale and T. mentagrophytes significantly reduced the thickness of the colonized membranes, whereas two T. rubrum strains showed the highest degradation limited to the small colonized area. These results were confirmed by SEM images of the colonization profile on membranes. Mechanical analyses performed on membranes were used as an innovative method to evaluate the thickness and structural integrity of membranes variation following fungal colonization. In conclusion, mechanical analyses of substrate may be used as a procedure for the development of a new onychomycosis diagnosis test in order to develop personalized and strain-specific treatment.
Keywords: Trichophyton; bovine nail membranes; dermatophytes; onychomycosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

