Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action
- PMID: 36354914
- PMCID: PMC9693873
- DOI: 10.3390/jof8111147
Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action
Abstract
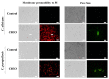
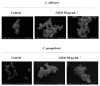
Antimicrobial drugs are becoming ineffective given the resistance acquired by microorganisms. As such, it is imperative to seek new antimicrobial molecules that could provide a basis for the development of new drugs. Therefore, this work aimed to evaluate the antimicrobial potential and the mechanisms of action of the essential oil extracted from leaves of Croton blanchetianus (named CbEO) on different fungi and bacteria of clinical importance in both planktonic and biofilm lifestyles. GC-MS/MS analysis revealed the presence of twenty-two different compounds in the CbEO, which were identified using the Kovats retention index. Among these, the most abundant were amorphene (20.03%), spathulenol (5%), bicyclogermacrene (1.49%), caryophyllene oxide (4.55%), and eucalyptol (5.62%). CbOE (50 µg mL-1) barely inhibited the growth of Bacillus subtilis (23%), Pseudomonas aeruginosa (27%), and Salmonella enterica (28%), and no inhibition was obtained against Enterobacter aerogenes and Klebsiella pneumoniae. Additionally, no activity against bacterial biofilm was detected. In contrast, CbEO was active against Candida species. C. albicans and C. parapsilosis were inhibited by 78 and 75%, respectively. The antibiofilm potential also was favorable against C. albicans and C. parapsilosis, inhibiting 44 and 74% of biofilm formation and reducing around 41 and 27% of the preformed biofilm, respectively. CbOE caused membrane damage and pore formation, overproduction of ROS, and apoptosis on C. albicans and C. parapsilosis cells, as well as not inducing hemolysis in human red cells. The results obtained in this work raise the possibility of using the essential oil of C. blanchetianus leaves as an alternative to fight infections caused by C. albicans and C. parapsilosis.
Keywords: Candida genus; GC-MS/MS; antibiofilm activity; biotechnological potential; essential oil.
Conflict of interest statement
The authors report no conflict of interest. The authors alone are responsible for the content and the writing of the paper.
Figures






References
-
- Do Vale J.P.C., Ribeiro L.H.d.F., de Vasconcelos M.A., Sá-Firmino N.C., Pereira A.L., do Nascimento M.F., Rodrigues T.H.S., da Silva P.T., de Sousa K.C., da Silva R.B., et al. Chemical Composition, Antioxidant, Antimicrobial and Antibiofilm Activities of Vitex gardneriana Schauer Leaves’s Essential Oil. Microb. Pathog. 2019;135:103608. doi: 10.1016/j.micpath.2019.103608. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

