New Theonellapeptolides from Indonesian Marine Sponge Theonella swinhoei as Anti-Austerity Agents
- PMID: 36354984
- PMCID: PMC9697519
- DOI: 10.3390/md20110661
New Theonellapeptolides from Indonesian Marine Sponge Theonella swinhoei as Anti-Austerity Agents
Abstract
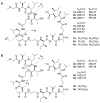
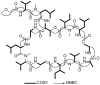
We reported three new members of the theonellapeptolide family from theonellapeptolide II series, namely theonellapeptolides IIb (1), IIa (2), IIc (3), and three known members-IId (4), IIe (5), and Id (6)-from Kodingarengan marine sponge Theonella swinhoei collected in Makassar, Indonesia. The structures of tridecadepsipeptides 1-3, including the absolute configurations of their amino acids, were determined by the integrated NMR and tandem MS analyses followed by Marfey's analysis. To the best of our knowledge, 1 and 2 are the first theonellapeptolide-type compounds to have a valine residue with D configuration at residue position 6. The isolated theonellapeptolide-type compounds 1-6 showed selective cytotoxic activity against human pancreatic MIA PaCa-2 cancer cells in a nutrient-deprived medium. Among them, the most potent preferential cytotoxicity was observed in new theonellapeptolide IIc (3) and known IId (4), IIe (5), and Id (6).
Keywords: Theonella swinhoei; anti-austerity agents; anti-pancreatic cancer agents; depsipeptide; marine sponge; preferential cytotoxicity; theonellapeptolide-type compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Fusetani N., Matsunaga S. Bioactive sponge peptides. Chem. Rev. 1993;93:1793–1806. doi: 10.1021/cr00021a007. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

