Discoidin domain receptor regulates ensheathment, survival and caliber of peripheral axons
- PMID: 36355066
- PMCID: PMC10112903
- DOI: 10.1242/dev.200636
Discoidin domain receptor regulates ensheathment, survival and caliber of peripheral axons
Abstract
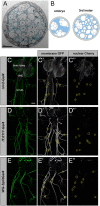
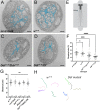
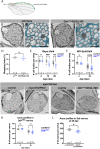
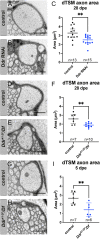
Most invertebrate axons and small-caliber axons in mammalian peripheral nerves are unmyelinated but still ensheathed by glia. Here, we use Drosophila wrapping glia to study the development and function of non-myelinating axon ensheathment, which is poorly understood. Selective ablation of these glia from peripheral nerves severely impaired larval locomotor behavior. In an in vivo RNA interference screen to identify glial genes required for axon ensheathment, we identified the conserved receptor tyrosine kinase Discoidin domain receptor (Ddr). In larval peripheral nerves, loss of Ddr resulted in severely reduced ensheathment of axons and reduced axon caliber, and we found a strong dominant genetic interaction between Ddr and the type XV/XVIII collagen Multiplexin (Mp), suggesting that Ddr functions as a collagen receptor to drive axon wrapping. In adult nerves, loss of Ddr decreased long-term survival of sensory neurons and significantly reduced axon caliber without overtly affecting ensheathment. Our data establish essential roles for non-myelinating glia in nerve development, maintenance and function, and identify Ddr as a key regulator of axon-glia interactions during ensheathment and establishment of axon caliber.
Keywords: Drosophila; Axon ensheathment; Multiplexin; Remak Schwann cell; Wrapping glia.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

