Esterified Oxylipins: Do They Matter?
- PMID: 36355090
- PMCID: PMC9697791
- DOI: 10.3390/metabo12111007
Esterified Oxylipins: Do They Matter?
Abstract
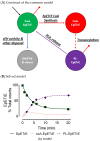
Oxylipins are oxygenated metabolites of fatty acids that share several similar biochemical characteristics and functions to fatty acids including transport and trafficking. Oxylipins are most commonly measured in the non-esterified form which can be found in plasma, free or bound to albumin. The non-esterified form, however, reflects only one of the possible pools of oxylipins and is by far the least abundant circulating form of oxylipins. Further, this fraction cannot reliably be extrapolated to the other, more abundant, esterified pool. In cells too, esterified oxylipins are the most abundant form, but are seldom measured and their potential roles in signaling are not well established. In this review, we examine the current literature on experimental oxylipin measurements to describe the lack in reporting the esterified oxylipin pool. We outline the metabolic and experimental importance of esterified oxylipins using well established roles of fatty acid trafficking in non-esterified fatty acids and in esterified form as components of circulating lipoproteins. Finally, we use mathematical modeling to simulate how exchange between cellular esterified and unesterified pools would affect intracellular signaling.. The explicit inclusion of esterified oxylipins along with the non-esterified pool has the potential to convey a more complete assessment of the metabolic consequences of oxylipin trafficking.
Keywords: esterification; lipoprotein; metabolism; oxylipin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Method optimization of oxylipin hydrolysis in nonprocessed bovine milk indicates that the majority of oxylipins are esterified.J Food Sci. 2021 May;86(5):1791-1801. doi: 10.1111/1750-3841.15697. Epub 2021 Apr 17. J Food Sci. 2021. PMID: 33864645
-
Targeting esterified oxylipins by LC-MS - Effect of sample preparation on oxylipin pattern.Prostaglandins Other Lipid Mediat. 2020 Feb;146:106384. doi: 10.1016/j.prostaglandins.2019.106384. Epub 2019 Nov 4. Prostaglandins Other Lipid Mediat. 2020. PMID: 31698140
-
Impact of circulating esterified eicosanoids and other oxylipins on endothelial function.Curr Atheroscler Rep. 2009 Nov;11(6):403-10. doi: 10.1007/s11883-009-0061-3. Curr Atheroscler Rep. 2009. PMID: 19852880 Review.
-
Clinical blood sampling for oxylipin analysis - effect of storage and pneumatic tube transport of blood on free and total oxylipin profile in human plasma and serum.Analyst. 2020 Mar 21;145(6):2378-2388. doi: 10.1039/c9an01880h. Epub 2020 Feb 10. Analyst. 2020. PMID: 32037406
-
Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids.Food Funct. 2017 Jul 19;8(7):2355-2367. doi: 10.1039/c7fo00403f. Food Funct. 2017. PMID: 28682409 Review.
Cited by
-
ABCA1 and apoA-I dependent 12-hydroxyeicosatetraenoic acid efflux regulates macrophage inflammatory signaling.bioRxiv [Preprint]. 2024 Jul 12:2024.07.11.603001. doi: 10.1101/2024.07.11.603001. bioRxiv. 2024. PMID: 39026807 Free PMC article. Preprint.
-
Stearidonic acid improves eicosapentaenoic acid status: studies in humans and cultured hepatocytes.Front Nutr. 2024 Apr 4;11:1359958. doi: 10.3389/fnut.2024.1359958. eCollection 2024. Front Nutr. 2024. PMID: 38974810 Free PMC article.
-
Identification of plasma proteins binding oxidized phospholipids using pull-down proteomics and OxLDL masking assay.J Lipid Res. 2025 Jan;66(1):100704. doi: 10.1016/j.jlr.2024.100704. Epub 2024 Nov 19. J Lipid Res. 2025. PMID: 39566852 Free PMC article.
-
How Membrane Phospholipids Containing Long-Chain Polyunsaturated Fatty Acids and Their Oxidation Products Orchestrate Lipid Raft Dynamics to Control Inflammation.J Nutr. 2024 Sep;154(9):2862-2870. doi: 10.1016/j.tjnut.2024.07.015. Epub 2024 Jul 25. J Nutr. 2024. PMID: 39025329 Free PMC article. Review.
-
Quantitative Profiling Method for Oxylipins in Neurodegenerative Diseases by Liquid Chromatography Coupled with Tandem Mass Spectrometry.bioRxiv [Preprint]. 2023 Oct 3:2023.10.02.560544. doi: 10.1101/2023.10.02.560544. bioRxiv. 2023. PMID: 37873260 Free PMC article. Preprint.
References
-
- Cho H.-J., Switzer C.H., Kamynina A., Charles R., Rudyk O., Ng T., Burgoyne J.R., Eaton P. Complex interrelationships between nitro-alkene-dependent inhibition of soluble epoxide hydrolase, inflammation and tumor growth. Redox Biol. 2019;29:101405. doi: 10.1016/j.redox.2019.101405. - DOI - PMC - PubMed
-
- Cole R.M., Puchala S., Ke J.-Y., Abdel-Rasoul M., Harlow K., O’Donnell B., Bradley D., Andridge R., Borkowski K., Newman J.W., et al. Linoleic Acid–Rich Oil Supplementation Increases Total and High-Molecular-Weight Adiponectin and Alters Plasma Oxylipins in Postmenopausal Women with Metabolic Syndrome. Curr. Dev. Nutr. 2020;4:nzaa136. doi: 10.1093/cdn/nzaa136. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

