ABHD5-A Regulator of Lipid Metabolism Essential for Diverse Cellular Functions
- PMID: 36355098
- PMCID: PMC9694394
- DOI: 10.3390/metabo12111015
ABHD5-A Regulator of Lipid Metabolism Essential for Diverse Cellular Functions
Abstract
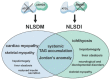
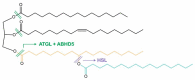
The α/β-Hydrolase domain-containing protein 5 (ABHD5; also known as comparative gene identification-58, or CGI-58) is the causative gene of the Chanarin-Dorfman syndrome (CDS), a disorder mainly characterized by systemic triacylglycerol accumulation and a severe defect in skin barrier function. The clinical phenotype of CDS patients and the characterization of global and tissue-specific ABHD5-deficient mouse strains have demonstrated that ABHD5 is a crucial regulator of lipid and energy homeostasis in various tissues. Although ABHD5 lacks intrinsic hydrolase activity, it functions as a co-activating enzyme of the patatin-like phospholipase domain-containing (PNPLA) protein family that is involved in triacylglycerol and glycerophospholipid, as well as sphingolipid and retinyl ester metabolism. Moreover, ABHD5 interacts with perilipins (PLINs) and fatty acid-binding proteins (FABPs), which are important regulators of lipid homeostasis in adipose and non-adipose tissues. This review focuses on the multifaceted role of ABHD5 in modulating the function of key enzymes in lipid metabolism.
Keywords: ABHD5; ATGL; CGI-58; Chanarin-Dorfman syndrome; NAFLD; NLSD; PNPLA3; ichthyosis; lipid metabolism; triglyceride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J.G., Gorkiewicz G., Zechner R. Adipose Triglyceride Lipase-Mediated Lipolysis of Cellular Fat Stores is Activated by CGI-58 and Defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. - DOI - PubMed
-
- Subramanian V., Rotlienberg A., Gomez C., Cohen A.W., Garcia A., Bhattacharyya S., Shapiro L., Dolios G., Wang R., Lisanti M.P., et al. Perilipin A Mediates the Reversible Binding of CGI-58 to Lipid Droplets in 3T3-L1 Adipocytes. J. Biol. Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

