Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders
- PMID: 36355147
- PMCID: PMC9692419
- DOI: 10.3390/metabo12111064
Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders
Abstract
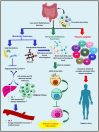
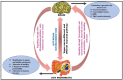
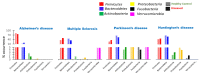
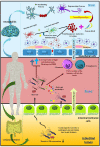
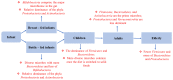
The gut-brain axis is a bidirectional communication network connecting the gastrointestinal tract and central nervous system. The axis keeps track of gastrointestinal activities and integrates them to connect gut health to higher cognitive parts of the brain. Disruption in this connection may facilitate various neurological and gastrointestinal problems. Neurodegenerative diseases are characterized by the progressive dysfunction of specific populations of neurons, determining clinical presentation. Misfolded protein aggregates that cause cellular toxicity and that aid in the collapse of cellular proteostasis are a defining characteristic of neurodegenerative proteinopathies. These disorders are not only caused by changes in the neural compartment but also due to other factors of non-neural origin. Mounting data reveal that the majority of gastrointestinal (GI) physiologies and mechanics are governed by the central nervous system (CNS). Furthermore, the gut microbiota plays a critical role in the regulation and physiological function of the brain, although the mechanism involved has not yet been fully interpreted. One of the emerging explanations of the start and progression of many neurodegenerative illnesses is dysbiosis of the gut microbial makeup. The present understanding of the literature surrounding the relationship between intestinal dysbiosis and the emergence of certain neurological diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and multiple sclerosis, is the main emphasis of this review. The potential entry pathway of the pathogen-associated secretions and toxins into the CNS compartment has been explored in this article at the outset of neuropathology. We have also included the possible mechanism of undelaying the synergistic effect of infections, their metabolites, and other interactions based on the current understanding.
Keywords: gut dysbiosis; gut microbiota; gut–brain axis; neurodegenerative disease; neuroinflammation; vagus nerve.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The authors have reported no potential conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

