Laminin N-terminus (LaNt) proteins, laminins and basement membrane regulation
- PMID: 36355367
- PMCID: PMC9788559
- DOI: 10.1042/BST20210240
Laminin N-terminus (LaNt) proteins, laminins and basement membrane regulation
Abstract
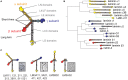
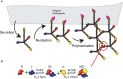
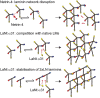
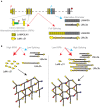
Basement membranes (BMs) are structured regions of the extracellular matrix that provide multiple functions including physical support and acting as a barrier, as a repository for nutrients and growth factors, and as biophysical signalling hubs. At the core of all BMs is the laminin (LM) family of proteins. These large heterotrimeric glycoproteins are essential for tissue integrity, and differences between LM family members represent a key nexus in dictating context and tissue-specific functions. These variations reflect genetic diversity within the family, which allows for multiple structurally and functionally distinct heterotrimers to be produced, each with different architectures and affinities for other matrix proteins and cell surface receptors. The ratios of these LM isoforms also influence the biophysical properties of a BM owing to differences in their relative ability to form polymers or networks. Intriguingly, the LM superfamily is further diversified through the related netrin family of proteins and through alternative splicing leading to the generation of non-LM short proteins known as the laminin N-terminus (LaNt) domain proteins. Both the netrins and LaNt proteins contain structural domains involved in LM-to-LM interaction and network assembly. Emerging findings indicate that one netrin and at least one LaNt protein can potently influence the structure and function of BMs, disrupting the networks, changing physical properties, and thereby influencing tissue function. These findings are altering the way that we think about LM polymerisation and, in the case of the LaNt proteins, suggest a hitherto unappreciated form of LM self-regulation.
Keywords: basement membrane; laminin; netrin.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

