Siderophore-Linked Ruthenium Catalysts for Targeted Allyl Ester Prodrug Activation within Bacterial Cells
- PMID: 36355416
- PMCID: PMC10108276
- DOI: 10.1002/chem.202202536
Siderophore-Linked Ruthenium Catalysts for Targeted Allyl Ester Prodrug Activation within Bacterial Cells
Abstract
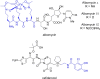
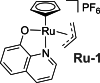
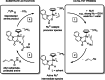
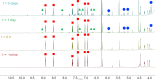
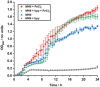
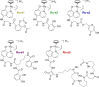
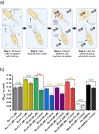
Due to rising resistance, new antibacterial strategies are needed, including methods for targeted antibiotic release. As targeting vectors, chelating molecules called siderophores that are released by bacteria to acquire iron have been investigated for conjugation to antibacterials, leading to the clinically approved drug cefiderocol. The use of small-molecule catalysts for prodrug activation within cells has shown promise in recent years, and here we investigate siderophore-linked ruthenium catalysts for the activation of antibacterial prodrugs within cells. Moxifloxacin-based prodrugs were synthesised, and their catalyst-mediated activation was demonstrated under anaerobic, biologically relevant conditions. In the absence of catalyst, decreased antibacterial activities were observed compared to moxifloxacin versus Escherichia coli K12 (BW25113). A series of siderophore-linked ruthenium catalysts were investigated for prodrug activation, all of which displayed a combinative antibacterial effect with the prodrug, whereas a representative example displayed little toxicity against mammalian cell lines. By employing complementary bacterial growth assays, conjugates containing siderophore units based on catechol and azotochelin were found to be most promising for intracellular prodrug activation.
Keywords: antibacterials; bio-orthogonal; catalysts; prodrugs; siderophores.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Rebelein J. G., Ward T. R., Curr. Opin. Biotechnol. 2018, 53, 106–114; - PubMed
- Ngo A. H., Bose S., Do L. H., Chem. Eur. J. 2018, 24, 10584–10594; - PubMed
- Martínez-Calvo M., Mascareñas J. L., Coord. Chem. Rev. 2018, 359, 57–79;
- van de L'Isle M. O. N., Ortega-Liebana M. C., Unciti-Broceta A., Curr. Opin. Chem. Biol. 2021, 61, 32–42; - PubMed
- Destito P., Vidal C., López F., Mascareñas J. L., Chem. Eur. J. 2021, 27, 4789–4816. - PubMed
-
- Völker T., Dempwolff F., Graumann P. L., Meggers E., Angew. Chem. Int. Ed. 2014, 53, 10536–10540; - PubMed
- Angew. Chem. 2014, 126, 10705–10710.
-
- Völker T., Meggers E., ChemBioChem 2017, 18, 1083–1086. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

