Syntaxin 4 is essential for hearing in human and zebrafish
- PMID: 36355422
- PMCID: PMC10026253
- DOI: 10.1093/hmg/ddac257
Syntaxin 4 is essential for hearing in human and zebrafish
Abstract
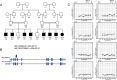
Congenital hearing impairment (HI) is a genetically highly heterogeneous disorder in which prompt recognition and intervention are crucial to optimize outcomes. In this study, we used exome sequencing to investigate a large consanguineous Pakistani family with eight affected individuals showing bilateral severe-to-profound HI. This identified a homozygous splice region variant in STX4 (c.232 + 6T>C), which causes exon skipping and a frameshift, that segregated with HI (two-point logarithm of odds (LOD) score = 5.9). STX4, a member of the syntaxin family, is a component of the SNARE machinery involved in several vesicle transport and recycling pathways. In silico analysis showed that murine orthologue Stx4a is highly and widespread expressed in the developing and adult inner ear. Immunofluorescent imaging revealed localization of STX4A in the cell body, cell membrane and stereocilia of inner and outer hair cells. Furthermore, a morpholino-based knockdown of stx4 in zebrafish showed an abnormal startle response, morphological and developmental defects, and a disrupted mechanotransduction function in neuromast hair cells measured via FM1-43 uptake. Our findings indicate that STX4 dysfunction leads to HI in humans and zebrafish and supports the evolutionary conserved role of STX4 in inner ear development and hair cell functioning.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Smith, R.J.H., Bale, J.F. and White, K.R. (2005) Sensorineural hearing loss in children. Lancet, 365, 879–890. - PubMed
-
- Van Camp, G. and Smith, R.J. Hereditary hearing loss homepage. Hereditary hearing loss homepage. https://hereditaryhearingloss.org/ (accessed 8 March 2022).
-
- Zazo Seco, C., Wesdorp, M., Feenstra, I., Pfundt, R., Hehir-Kwa, J.Y., Lelieveld, S.H., Castelein, S., Gilissen, C., de Wijs, I.J., Admiraal, R.J. et al. (2017) The diagnostic yield of whole-exome sequencing targeting a gene panel for hearing impairment in The Netherlands. Eur. J. Hum. Genet., 25, 308–314. - PMC - PubMed

