Identification of a neutrophil-specific PIK3R1 mutation facilitates targeted treatment in a patient with Sweet syndrome
- PMID: 36355435
- PMCID: PMC9797331
- DOI: 10.1172/JCI162137
Identification of a neutrophil-specific PIK3R1 mutation facilitates targeted treatment in a patient with Sweet syndrome
Abstract
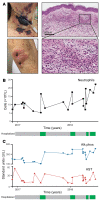
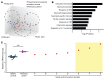
BackgroundAcute febrile neutrophilic dermatosis (Sweet syndrome) is a potentially fatal multiorgan inflammatory disease characterized by fever, leukocytosis, and a rash with a neutrophilic infiltrate. The disease pathophysiology remains elusive, and current dogma suggests that Sweet syndrome is a process of reactivity to an unknown antigen. Corticosteroids and steroid-sparing agents remain frontline therapies, but refractory cases pose a clinical challenge.MethodsA 51-year-old woman with multiorgan Sweet syndrome developed serious corticosteroid-related side effects and was refractory to steroid-sparing agents. Blood counts, liver enzymes, and skin histopathology supported the diagnosis. Whole-genome sequencing, transcriptomic profiling, and cellular assays of the patient's skin and neutrophils were performed.ResultsWe identified elevated IL-1 signaling in lesional Sweet syndrome skin caused by a PIK3R1 gain-of-function mutation specifically found in neutrophils. This mutation increased neutrophil migration toward IL-1β and neutrophil respiratory burst. Targeted treatment of the patient with an IL-1 receptor 1 antagonist resulted in a dramatic therapeutic response and enabled a tapering off of corticosteroids.ConclusionDysregulated PI3K/AKT signaling is the first signaling pathway linked to Sweet syndrome and suggests that this syndrome may be caused by acquired mutations that modulate neutrophil function. Moreover, integration of molecular data across multiple levels identified a distinct subtype within a heterogeneous disease that resulted in a rational and successful clinical intervention. Future patients will benefit from efforts to identify potential mutations. The ability to directly interrogate the diseased skin allows this method to be generalizable to other inflammatory diseases and demonstrates a potential personalized medicine approach for patients with clinically challenging disease.Funding SourcesBerstein Foundation, NIH, Veterans Affairs (VA) Administration, Moseley Foundation, and H.T. Leung Foundation.
Keywords: Dermatology; Inflammation; Neutrophils; Signal transduction; Skin.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

