Neuroprotective Effect of Red Sea Marine Sponge Xestospongia testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models
- PMID: 36355482
- PMCID: PMC9693000
- DOI: 10.3390/ph15111309
Neuroprotective Effect of Red Sea Marine Sponge Xestospongia testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models
Abstract
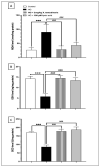
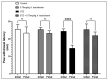
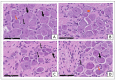
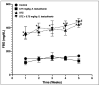
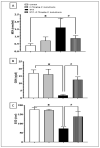
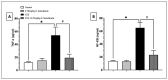
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes. Oxidative stress plays an important role in the pathophysiology of DPN. Red Sea marine sponge Xestospongia testudinaria extract has a promising neuroprotective effect, presumably owing to its antioxidant and anti-inflammatory properties. Thus, this study aimed to investigate the neuroprotective effect of the sponge X. testudinaria extract on in vitro and in vivo models of DPN. Mice dorsal root ganglia (DRG) were cultured with high glucose (HG) media and used as an in vitro model of DPN. Some of the DRGs were pre-treated with 2 mg/mL of X. testudinaria. The X. testudinaria extract significantly improved the HG-induced decreased neuronal viability and the neurite length. It improved the oxidative stress biomarkers in DRG cultures. The DPN model was induced in vivo by an injection of streptozotocin at a dose of 150 mg/kg in mice. After 35 days, 0.75 mg/kg of the X. testudinaria extract improved the hot hyperalgesia and the DRG histology. Although the sponge extract did not reduce hyperglycemia, it ameliorated the oxidative stress markers and pro-inflammatory markers in the DRG. In conclusion, the current study demonstrates the neuroprotective effect of Red Sea sponge X. testudinaria extract against experimentally induced DPN through its antioxidant and anti-inflammatory mechanisms.
Keywords: Red Sea marine sponge; Xestospongia testudinaria; anti-inflammatory; antioxidant; diabetic peripheral neuropathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Skapare E., Konrade I., Liepinsh E., Strele I., Makrecka M., Bierhaus A., Lejnieks A., Pirags V., Dambrova M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J. Diabetes Complicat. 2013;27:262–267. doi: 10.1016/j.jdiacomp.2012.12.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

