Reactivity of Covalent Fragments and Their Role in Fragment Based Drug Discovery
- PMID: 36355538
- PMCID: PMC9694498
- DOI: 10.3390/ph15111366
Reactivity of Covalent Fragments and Their Role in Fragment Based Drug Discovery
Abstract
Fragment based drug discovery has long been used for the identification of new ligands and interest in targeted covalent inhibitors has continued to grow in recent years, with high profile drugs such as osimertinib and sotorasib gaining FDA approval. It is therefore unsurprising that covalent fragment-based approaches have become popular and have recently led to the identification of novel targets and binding sites, as well as ligands for targets previously thought to be 'undruggable'. Understanding the properties of such covalent fragments is important, and characterizing and/or predicting reactivity can be highly useful. This review aims to discuss the requirements for an electrophilic fragment library and the importance of differing warhead reactivity. Successful case studies from the world of drug discovery are then be examined.
Keywords: covalent fragments; electrophilic warhead; fragment library; fragment-based drug discovery; reactivity.
Conflict of interest statement
There are no conflicts to declare.
Figures
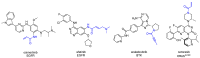
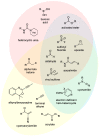
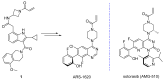
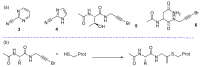
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

