Exciton Chirality Inversion in Dye Dimers Templated by DNA Holliday Junction
- PMID: 36355575
- PMCID: PMC9706552
- DOI: 10.1021/acs.jpclett.2c02721
Exciton Chirality Inversion in Dye Dimers Templated by DNA Holliday Junction
Abstract
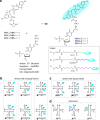
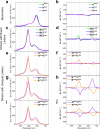
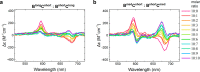
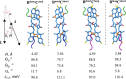
While only one enantiomer of chiral biomolecules performs a biological function, access to both enantiomers (or enantiomorphs) proved to be advantageous for technology. Using dye covalent attachment to a DNA Holliday junction (HJ), we created two pairs of dimers of bis(chloroindolenine)squaraine dye that enabled strongly coupled molecular excitons of opposite chirality in solution. The exciton chirality inversion was achieved by interchanging single covalent linkers of unequal length tethering the dyes of each dimer to the HJ core. Dimers in each pair exhibited profound exciton-coupled circular dichroism (CD) couplets of opposite signs. Dimer geometries, modeled by simultaneous fitting absorption and CD spectra, were related in each pair as nonsuperimposable and nearly exact mirror images. The origin of observed exciton chirality inversion was explained in the view of isomerization of the stacked Holliday junction. This study will open new opportunities for creating excitonic DNA-based materials that rely on programmable system chirality.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Exciton Delocalization in Indolenine Squaraine Aggregates Templated by DNA Holliday Junction Scaffolds.J Phys Chem B. 2020 Oct 29;124(43):9636-9647. doi: 10.1021/acs.jpcb.0c06480. Epub 2020 Oct 14. J Phys Chem B. 2020. PMID: 33052691 Free PMC article.
-
Photocrosslinking Probes Proximity of Thymine Modifiers Tethering Excitonically Coupled Dye Aggregates to DNA Holliday Junction.Molecules. 2022 Jun 22;27(13):4006. doi: 10.3390/molecules27134006. Molecules. 2022. PMID: 35807250 Free PMC article.
-
Effect of hydrophilicity-imparting substituents on exciton delocalization in squaraine dye aggregates covalently templated to DNA Holliday junctions.Nanoscale. 2024 Jan 18;16(3):1206-1222. doi: 10.1039/d3nr04499h. Nanoscale. 2024. PMID: 38113123
-
The enduring legacy of Koji Nakanishi's research on natural products and bioorganic chemistry. Part 2. Inception and establishment of the ECD exciton chirality method in 1960s to 1970s: A marvel of Nakanishi's Japanese team.Chirality. 2020 May;32(5):535-546. doi: 10.1002/chir.23193. Epub 2020 Mar 6. Chirality. 2020. PMID: 32142193 Review.
-
Supramolecular exciton chirality of carotenoid aggregates.Chirality. 2003 Oct;15(8):680-98. doi: 10.1002/chir.10282. Chirality. 2003. PMID: 12923806 Review.
Cited by
-
Pursuing excitonic energy transfer with programmable DNA-based optical breadboards.Chem Soc Rev. 2023 Nov 13;52(22):7848-7948. doi: 10.1039/d0cs00936a. Chem Soc Rev. 2023. PMID: 37872857 Free PMC article. Review.
-
Tunable and robust optical and structural properties of a cooperative squaraine-dye aggregate-DNA DX-DAE tile system.Nanoscale. 2025 Aug 15;17(32):18646-18677. doi: 10.1039/d5nr00863h. Nanoscale. 2025. PMID: 40748332 Free PMC article.
-
Towards tunable exciton delocalization in DNA Holliday junction-templated indodicarbocyanine 5 (Cy5) dye derivative heterodimers.Nanoscale Horiz. 2024 Nov 19;9(12):2334-2348. doi: 10.1039/d4nh00225c. Nanoscale Horiz. 2024. PMID: 39320147 Free PMC article.
-
Electronic Structure and Excited-State Dynamics of DNA-Templated Monomers and Aggregates of Asymmetric Polymethine Dyes.J Phys Chem A. 2023 Jun 15;127(23):4901-4918. doi: 10.1021/acs.jpca.3c00562. Epub 2023 Jun 1. J Phys Chem A. 2023. PMID: 37261888 Free PMC article.
-
Exciton delocalization in a fully synthetic DNA-templated bacteriochlorin dimer.Phys Chem Chem Phys. 2023 Oct 25;25(41):28437-28451. doi: 10.1039/d3cp01634j. Phys Chem Chem Phys. 2023. PMID: 37843877 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

