Exciton Chirality Inversion in Dye Dimers Templated by DNA Holliday Junction
- PMID: 36355575
- PMCID: PMC9706552
- DOI: 10.1021/acs.jpclett.2c02721
Exciton Chirality Inversion in Dye Dimers Templated by DNA Holliday Junction
Abstract
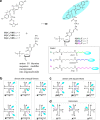
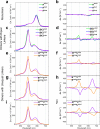
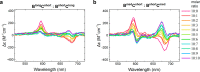
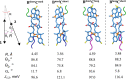
While only one enantiomer of chiral biomolecules performs a biological function, access to both enantiomers (or enantiomorphs) proved to be advantageous for technology. Using dye covalent attachment to a DNA Holliday junction (HJ), we created two pairs of dimers of bis(chloroindolenine)squaraine dye that enabled strongly coupled molecular excitons of opposite chirality in solution. The exciton chirality inversion was achieved by interchanging single covalent linkers of unequal length tethering the dyes of each dimer to the HJ core. Dimers in each pair exhibited profound exciton-coupled circular dichroism (CD) couplets of opposite signs. Dimer geometries, modeled by simultaneous fitting absorption and CD spectra, were related in each pair as nonsuperimposable and nearly exact mirror images. The origin of observed exciton chirality inversion was explained in the view of isomerization of the stacked Holliday junction. This study will open new opportunities for creating excitonic DNA-based materials that rely on programmable system chirality.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

